The abilities of skeleton to articulate in various opposing directions and to serve as levers for muscle action are fundamental to mobility and locomotion in vertebrates. Skeleton also plays important roles in maintaining blood calcium levels, supporting haematopoiesis, and housing the brain and the spinal cord [1]. Consequently, skeletal defects often incur considerable morbidity. Conventional medical strategies have barely cured irreversible tissue defects, focusing on the removal of causes of diseases. For the repair of tissue defects, they mostly rely on natural healing abilities of tissues, helping the abilities to be exerted efficiently. In bone and cartilage, irreversible tissue defects are caused by aging, trauma, disease, tumor, and developmental abnormalities.
Bone grafts and prosthetic implant devices have been used to repair irreversible bone defects. Autograft is superior to the other techniques in function and engraftment, because it is a bone tissue derived from the same individual containing live cells and growth factors. Autograft is thought to have both the ability to facilitate bone regeneration (osteoconductivity) and the ability to actively induce bone regeneration (osteoinductivity) and is speedily fused and integrated to the bone of the implantation site. However, the donor site morbidity often occurs, because autogaft requires highly-invasive bone collection surgery from healthy sites [2]. Although allograft is free from the invasiveness and less restricted in quantity, it runs a biological risk of contamination by pathogens as well as an ethical risk [3]. In allograft, no live cells are present, and growth factors are inactivated to some extent, since it is usually heat-treated and kept frozen in order to reduce immunological reactions. Therefore, its osteoconductivity and osteoinductivity are inferior to those of autograft.
Several strategies for the treatment of cartilage defects have been reported: autograft of periosteum and perichondrium, cartilage transplantation, and mechanical penetration of subchondral bones for the bone marrow entry into the defect site. However, they failed to provide reproducible results or complete repair of the defects [4]. In addition, common to the procedures for both bone and cartilage defects, physicians have to manually carve grafts to fit them to deformities during surgery, which is often time-consuming and laborious and associated with low precision [5]. Thus, grafts have short-comings concerning both quantity (availability of suitable graft material) and quality (donor site troubles, graft rejection and disease transmission). Prosthetic implants overcome some problems associated with grafts, but have shortcomings concerning biocompatibility, function, and longevity.
Tissue engineering of bone and cartilage has drawn attention as an approach which provides solutions to such problems. Back in 1993, Langer and Vacanti [6] proposed three components for the creation of new tissues: cell sources, tissue-inducing factors (signaling factors), and scaffolds. To bring tissue engineering into reality, it is crucial to justify and optimize the use of each component as well as to sufficiently advance and combine the three components [7].
This paper aims to review current progress on tissue engineering of bone and cartilage, focusing on important translational studies as well as preclinical studies. We also discuss major obstacles and future perspectives in this field. According to the three components for tissue engineering, the review is composed of the following sections:
Substantial evidences have been accumulated in the past few years to understand major osteogenic signaling molecules and genes: bone morphogenetic proteins (BMPs) [8], Hedgehogs (Hh) [9,10], runt-related transcription factor 2 (Runx2) [11,12], Wnts [13], fibroblast growth factors (FGFs) [14] (ref) and insulin-like growth factors (IGFs) [15]. Among them, BMPs have been most extensively studied in clinical settings. Efficacy of recombinant human BMP-2 (rhBMP-2) and rhBMP-7 in fracture repair of tibia and spine fusion has been shown by several clinical trials [16]. However, a large amount of BMP is required for the treatments, and BMP-containing devices often fail, raising concerns over costs and safety [17-19]. The reasons may be related to a lack of delivery systems which enables the release of BMPs in a controlled- and sustained-manner, short biological half-life of BMPs, and difficulties to mimic the biological condition [20]. The efficacy of FGF-2 on bone repair was also examined in clinical settings as well as in preclinical studies. In a randomized and placebo-controlled trial, Kawaguchi et al. revealed that a local application of gelatin hydrogel containing rhFGF-2 accelerated healing of tibial shaft fracture without any significant difference in the profiles of adverse events between treatment and control groups [21,22]. With regards to transcription factors, in vitro and preclinical studies point to Runx2 as a useful factor for bone regeneration using stem cells, osteoblast lineage cells, or cell populations containing either one [23-26].
Because the above studies focused on a single factor, the possibility still remained that other signaling molecules besides BMPs and Runx2 or those combinations might induce bone regeneration more potently than a single factor. Optimization of osteogenic signaling molecules through comprehensive screening addressed the concern. We screened cDNA libraries and the combination of activators or inhibitors of osteogenesis-related pathways (BMP, Hh, Runx2, Wnt, and IGF-1), and found that the combination of BMP signaling and Runx2 was the most potent for osteogenic differentiation. The combination induced the differentiation in mouse embryonic stem (ES) cells, human dermal fibroblasts, and non-osteogenic cell lines. We also succeeded in inducing rapid bone regeneration by transplantation of a monolayer sheet of fibroblasts transduced with the combination [27].
Another strategy for bone regeneration is to activate osteogenic signaling pathways by small chemical compounds. Statins [28], isoflavone derivatives [29,30], and TAK-778 [31] were reported to stimulate osteogenic differentiation, but their osteogenic activity was shown only in specific cell types including osteoblastic cells and stem cells. We have identified a couple of osteogenic small compounds including 4-(4-methoxyphenyl)pyrido[4',3':4,5]thieno[2,3-b] pyridine-2-carboxamide (TH) [32], icariin isolated from the herb Epimedium pubescens [33], and an isoflavone derivative, glabrisoflavone [34]. These compounds may be candidates for small compound-mediated bone regeneration in the future.
A number of chondrogenic factors have been clarified: sex determining region Y-type high mobility group box (Sox) 5/6/9 [35], IGF-1 [36], FGF-2 [37], Hhs [38], BMP-2 [39], transforming growth factor β (TGF-β) [40], and Wnts [38]. Indrawattana et al. [41] reported the use of three factors, TGF-β3, BMP-6 and IGF-1 in pellet cultures of human bone marrow cells for chondrogenic induction. IGF-1-loaded fibrin clots induced cartilage repair in critical-sized, full thickness defects in adult horses [42] and partial thickness ones in mini pigs [43]. Implantation of chondrocytes loaded on IGF-1-containing fibrin clots improved the overall continuity and consistency of the cartilage repair, as compared with that of chondrocytes alone, in the horse model [44]. TGF-β1 was shown to repair a full-thickness cartilage defect by improving chondrocyte integration into the endogenous tissue and to induce the differentiation of MSCs to form ectopic cartilage in vivo [45]. Regarding FGFs, Ishii et al. [46] reported that the implantation of the fibrin sealant incorporating FGF-2 successfully induced healing of the surface with hyaline cartilage and concomitant repair of the subchondral bone in cartilage defects in rabbits' knees. FGF18 also stimulated repair of damaged cartilage [47].
To identify potent combination of chondrogic factors, we isolated ES cells from transgenic mice expressing green fluorescent protein (GFP) under the control of a Col2a1 promoter region. The cells were expected to fluoresce solely upon chondrogenic differentiation. Using this system, we examined the effects of gain and loss of function of representative factors that are known to be important for chondrogenesis: SOX5, SOX6, SOX9, IGF-1, FGF-2, Indian hedgehog, BMP-2, TGF-β, and Wnts. GFP expression was observed only upon treatment with the SOX 5/6/9 (SOX trio). The SOX trio successfully induced chondrocyte differentiation in all cell types tested, including ES cells, mesenchymal stromal cells (MSCs), and human skin fibroblasts. Contrary to the conventional chondrogenic techniques, the SOX trio suppressed hypertrophic and osteogenic differentiation at the same time [48]. However, there was still room for improvement in matrix synthesis.
Aiming at the clinical application of autologous chondrocytes to cartilage regeneration, the combination of growth factors was optimized to expand human chondrocytes and to re-differentiate de-differentiated chondrocyes in culture [49,50]. The combination of FGF-2 with insulin or IGF-I was suggested to be useful for promotion of chondrocyte proliferation [49]. Also, the combination of BMP-2, insulin, and triiodothyronine was found to be the most effective for the re-differentiation of the de-differentiated cells after repeated passages [50].
In bone and cartilage tissue engineering, autologous cell transplantation of MSCs derived from bone marrow (bone marrow MSCs) has been widely used. The website of United States National Institute of Health (http://www.clinicaltrials.gov) discloses that several clinical trials are ongoing to evaluate the safety and/or efficacy of autologous MSC transplants in bone and cartilage defects. Bone marrow MSCs were initially identified as bone marrow-derived adherent cells proliferating in culture and having a property of bone and cartilage progenitors [51,52]. The cells have been reported to have abilities to differentiate into nerve cells [53] and hepatocytes [54] as well as cells derived from mesenchyme such as osteoblasts, chondrocytes, adipocytes, and muscle cells [55,56]. When infused into children with osteogenesis imperfecta, bone marrow MSCs induced new lamellar bone formation and an increase in the total body mineral content with the increased number of osteoblasts [57,58]. Quarto et al. [59] described treatment of patients with large bone defects with autologous MSCs in combination with porous ceramic scaffolds. In the treatment of non- or delayed union, clinical studies have been performed to examine the efficacy of bone marrow cells [16].
As for the use of autologous MSCs in cartilage defects, Wakitani et al. and Kuroda et al. [60-63] reported cartilage repair in human by the implantation of autologous bone marrow MSC-containing collagen gels into knees. To examine the possibility that MSCs might form tumors during long-term follow-up, they further investigated tumor development and infections in 41 patients who had undergone autologous bone marrow implantation for cartilage repair. In the follow-up ranging from 5 to 137 months (11 years and 5 months), they found that none of the patients had infections or tumors [64].
Synovium has also drawn attention as another source of MSCs. A couple of studies have shown that MSCs isolated from synovium (synovial MSCs) show higher chondrogenic capacity than MSCs isolated from other tissues. Sakaguchi et al. [65] found in vitro that human synovial MSCs had higher capacity to differentiate into chondrocytes than MSCs derived from any other tissues including bone marrow, periosteum, muscle, and adipose tissue. Synovial MSCs induced cartilage repair more potently than muscle- or adipose tissue-derived ones in a rat cartilage defect model [66]. Moreover, synovial MSCs were reported to expand more than bone marrow MSCs with autologous human serum in vitro [67]. These data suggest that synovial MSCs are a more potent cell source for cartilage repair than bone marrow MSCs. However, isolating MSC from synovium is more difficult than that from bone marrow, which is a reason why bone marrow MSCs have been used more widely [68].
MSCs still have technical limitations both in quantity and differentiation capacity. More than 109 cells are supposed to be required for the treatment of clinical bone defects [69]. However, only 103 to 106 cells can be isolated from 10 mL of bone marrow fluid or adipose tissue [69,70], and it is difficult to expand MSCs by several rounds of passages without affecting their differentiation capacity [71].
On the other hand, embryonic stem cells (ESCs) proliferate practically indefinitely and possess pluripotency (an ability to give rise to all cell types of embryo) [72]. The cells, isolated from the inner cell mass of blastcysts, were initially established from mouse embryo in 1980s [73,74] and later in 1998 from human [75]. In vitro studies have shown that human and mouse ES cells can differentiate into osteoblatic cells under certain conditions. In a basic differentiation protocol [76], embryoid bodies (EBs) derived from ESCs are cultured in an osteogenic medium containing dexamethazone, beta glycerophosphate, and ascorbic acid, which was originally developed for osteogenic induction of bone marrow MSCs and primary osteoblasts [77]. The protocol has been modified with the addition of BMP-2 [78], BMP-4 [79], compactin [78], vitamin D3 [80], or Leucine-rich amelogenin peptide [81]. Compared with substantial evidences for in vitro osteogenic differentiation of ESCs, their capacities of in vivo bone formation appeared to be poor [82]. Jukes et al. [83] reported in 2008 that they successfully achieved bone regeneration in rat bone defect model using mouse ESCs by mimicking the process of endochondral ossification, where cartilage first formed and then it was replaced by bone tissues.
Mouse ESCs are likely to have the ability to spontaneously differentiate into chondrocytic cells, since cartilageous tissues were frequently observed in teratomas which arose from undifferentiated ESCs implanted in vivo. Consistent with the in vivo findings, Kramer et al. [84] reported that cartilageous tissues that appeared to be in various stages of chondrogenesis were formed in mouse EBs when they were cultured on tissue culture plastic with basic ES culture media. To further enhance the differentiation efficiency, serum-free chondrogenic media supplemented with several growth factors have been examined. The media, originally established in MSCs, contained insulin, transferring, selenious acid insulin, transferrin, and selenious acid, dexamethasone, ascorbic acid, sodium pyruvate, proline, and TGF-β1 or TGF-β3 [82]. Co-cultures with limb bud cells or chondrocytes enhanced chondrogenic differentiation of ESCs [85-87]; direct cell-cell contact was important for chondrogenic differentiation of ESCs in co-culture of ESCs with limb bud cells, whereas co-culture with chondrocytes did not require the direct contact. Tanaka et al. [88] found that chondrogenic differentiation of ESCs was enhanced in the three-dimensional culture. Oldershaw et al. [89] recently reported a protocol to differentiate human ESCs into chondrocytes by driving the differentiation through primitive streak-mesoderm and mesoderm intermediates to chondrocytes, which was a sequence of chondrocyte formation in development. It is noteworthy that they achieved the highly-efficient differentiation using chemically-defined media supplemented with known growth factors.
Despite these extensive studies performed so far, four major problems remain to be solved for the use of ESCs in tissue engineering. The first is low efficiency of differentiation protocols. The second is teratoma formation by residual undifferentiated cells. The third is immunological reaction. The fourth is ethical issues accompanying the use of human embryo. Although the immunological and ethical problems of ESCs may be solved by induced pluripotent stem cells (iPSCs) to some extent, problems concerning low differentiation efficiency and teratoma formation still remain. For the osteogenic differentiation of iPSCs, protocols established in ESCs have been modified. Overexpression of Runx2 [90], a master regulator of osteoblast differentiation, and treatment with resveratrol [91], a polyphenol antioxidant, enhanced in vitro osteogenic differentiation of iPSCs. Subcutaneous implantation of a gelfoam matrix containing iPSC-derived osteoblasts induced bone formation with vasculature recruitment [92]. Ye et al. [93] achieved in vivo bone regeneration in a mouse calvarial bone defect model, by transplantation of iPSCs overexpressing SATB2, a nuclear matrix protein promoting osteogenesis by interacting with Runx2 and activating transcription factor 4 [93]. They claimed that no tumor development was observed in any of mice that had undergone the transplantation of SATB2-overexpressing iPSCs. However, to apply this methods to clinical settings, it is necessary to examine how many cells are still in an undifferentiated state and completely sort out them, if any, after the osteogenic induction with SATB2. Chondrogenic ability of iPSCs was also tested using differentiation protocols established in ESCs [94].
Utilizing abundant autologous adult cells such as skin fibroblasts may overcome problems associated with the use of stem cells. We and others have shown that skin fibroblasts can be a cell source for bone and cartilage regeneration. Hirata et al. [95] and Krebsbach et al. [96] described in vivo bone regeneration using dermal fibroblasts that were infected with adenoviruses expressing BMP-7 and BMP-2, respectively. We reported the efficacy of optimized osteogenic signal on in vivo bone regeneration using mouse dermal fibroblasts [27] and the induction of chondrocyte markers in human skin fibroblasts in vitro by overexpressing the SOX trio [48]. Because the fibroblast-derived chondrocytes appeared to form fibrocartilage rather than hyaline cartilage, Hiramatsu et al. [97] hypothesized that type I collagen expression still persisted in the cells and reprogramming factors might eliminate fibroblastic properties during chondrogenic differentiation of fibroblasts. Indeed, they achieved the generation of hyaline cartilage with fibroblasts retrovirally-infected with two reprogramming factors, c-Myc and Klf4, and Sox9 [97].
In bone tissue engineering, three biomaterials (metals, ceramics, and polymers) have been widely used. Titanium, a traditional inert biomaterial for implants, is characterized by a minimal immune response, which is the biggest advantage of this material. Some studies have shown that titanium fiber meshes or titanium with Zinc-containing hydroxyapatite enhance the osteogenic activity or the proliferation of seeded cells [98-100]. However, Tortelli and Cancedda [101] pointed out that the difficulty in performing histological analyses was a serious drawback in further investigating the biological activity of this material.
Hydroxyapatite- and beta-tricalcium phosphate (beta-TCP)-based scaffolds are widely used, and several different bioceramics have been developed in order to improve their properties [102-105]. In particular, calcium phosphates are the most popular materials for artificial bones [106,107]. Their biocompatibility and biosafety are, in a sense, already tested in the living body, since approximately 70% of bone of our body is made of the calcium phosphates [108]. The calcium phosphates are known to be naturally osteoconductive [108] and metabolized and degraded by the endogenous bone remodeling system. Thus, the artificial bones made of the calcium phosphates are superior to autograft and allograft, in terms of biosafety, unlimited quantity, and low invasiveness. Given that interconnectivity of the pores was the primary determinant for osteoconductivity, Tamai et al. [109] developed a fully interconnected porous calcium hydroxyapatite ceramics by a foam-gel technique. We also developed a novel tetrapod-shape granular artificial bone using micro-particles of alpha-TCP by injection molding, (manuscript in preparation). When stuffed in a space, the artificial bones were proven to form more effective intergranular pores for cell and vascular invasion because of their homogeneous shape and size.
The artificial bones usually require post-fabrication sintering process to increase their mechanical properties, which causes contraction in size and often decreases biodegradability, as well as shape adjustment during surgery [110-113]. Therefore, there is a need for novel artificial bones that have better shape compatibility to deformities, appropriate mechanical strength without the post-fabrication sintering, and biodegradability. By controlling the three dimentional (3D) shape of the scaffolds, we have developed artificial bones with good dimensional compatibility, which results in reduction in the operation time, invasiveness, and speedy union with the host bone tissues [114-116]. Oka et al. [117] also reported the use of customized hydroxyapatite implants which had preoperatively cut into a shape, based on a computer simulation with 3D-computed tomography (CT) data, in opening wedge osteotomy.
Biodegradable synthetic polymers applied to bone tissue engineering include poly (lactic acid) (PLA), poly (glycolic acid) (PGA), poly (-caprolactone), and poly (lactic-coglycolide) (PLGA) copolymers [118-126]. Cellular adhesion to PLGA is significantly higher than on PLA surfaces, and PLGA scaffolds were shown to induce osteoblast proliferation and differentiation. PLGA supported proliferation and differentiation of osteoblasts, as shown by high alkaline phosphatase activity and deposition of a mineralized matrix [127,128]. PLGA has been also utilized for encapsulation and release of several growth factors including TGF-β, BMPs, IGFs, vascular endothelial growth factor, and nerve growth factor. Several studies have tested variable sized PLGA microspheres with growth factors and subsequent embedding of them in other polymer matrices with variable degradation rates [129]. Nie and Wang [130] reported the delivery of plasmids expressing BMP-2 using PLGA/Hydroxyapatite composite scaffolds. Takaoka and their colleagues studied PLA derivatives and their composites with other materials as a carrier of rhBMP-2 [131-134]. They combined a block co-polymer of PLA-p-dioxanone-poly (ethylenglycol) (PEG) and beta-TCP (PLA/PEG/beta-TCP) [135]. The efficacy of the rhBMP-2-loaded PLA/PEG/beta-TCP in bone repair has been shown by various animal models relevant to clinical situations [136-139]. Although Poly (ε-caprolactone) carries biocompatibility and processability, it is less suitable for long term applications because of its high hydrophobicity and low degradability in vivo [140].
Among natural polymers, bovine type I collagen has been used as a promising biomaterial. Several type I collagen-based materials are commercially available including CollapatII (Biomet Inc., Warsaw, IN, USA), Healos (Depuy Spine Inc., Raynham, MA, USA), Collagraft (Neucoll Inc., Palo Alto, CA, USA; Zimmer Inc., Warsaw, IN, USA) and Biostite (Vebas S.r.l., San Giuliano Milanese, Italy) [141]. Given that collagens are dominant and important matrix in bone tissues, it makes sense that the scaffold has biocompatibility as well as the activity to facilitate osteogenesis or cell proliferation. However, two major concerns, disease transmission from other species and its poor mechanical property, remain to be solved.
Scaffolds for cartilage tissue engineering are classified into three groups: polysaccharide-based natural, protein-based natural, and synthetic biomaterials. Polysaccharide-based materials include alginate, chitosan, cellurose, and hyaluronic acid [4]. Hyalograft C, the combination of a hyaluronic-acid-based matrix HYAFF-11 (Fidia Advanced Biopolymers, Abano Terme, Italy), and autologous chondrocytes, is used in clinical settings [142].
Among protein-based materials, bilayer collagen type I and III membranes are clinically available for autologous chondrocyte implantation (ACI): MACI (Matrix-induced ACI; Verigen, Leverkusen, Germany), Maix (Matricel, Hezoenrath, Germany) and Chondrogide (Geistlich Biomaterials, Wolhusen, Switzerland) [4]. Atelocollagen (Koken Co., Ltd., Tokyo, Japan) is a type I collagen gel, from which telopeptide causing antigenecity is removed. The material has an advantage in generating 3D structure of implant. Ochi et al. [143,144] have applied the aterocollagen as a carrier of chondrocytes in ACI. However, when implanted, the ateloacollagen-cell composites should be covered with periosteum not to be detached from implantation sites [145]. In addition, fibrin glue (Tissucol, BAXTER, Vienna, Austria) was used for cartilage repair, and its 1-year clinical results were reported by Visna et al. [146].
PEG is used to create synthetic-based hydrogels in cartilage tissue engineering. Chondrocytes remained viable and synthesized cartilage-specific extracellular matrix, even when they were encapsulated in a PEG hydrogel under a compressive modulus (i.e., 260-900 kPa) similar to that of human cartilage (i.e., 790 ± 360 kPa) [147,148]. Bio-Seed-C (BioTissue Technologies, Freiburg, Germany) is a clinically-available synthetic material, in which a porous 3D scaffold consisting of PGA, PLA, and polydioxanone is combined with autologous chondrocytes embedded in fibrin gel. Bio-Seed1-C (BioTissue Technologies) induced the formation of hyaline cartilage and a significant clinical improvement of joint function [149].
Since human multipotent MSCs were isolated, expanded, and characterized in the late 1990s [56], researchers in this field have explored suitable ways to apply the cells to tissue engineering of bone and cartilage. Despite a large number of preclinical and clinical studies providing several promising results, we still struggle with finding ways to utilize stem cells more safely and effectively. Although no one disputes that stem cells are a promising cell source for tissue regeneration, we need to keep in mind that stem cells are not necessarily a panacea. It is about time to think if the use of stem cells is really required for all cases with irreversible skeletal defects. In bone tissue engineering, for example, it appears possible to repair some bone defects without cell transplantation by inducing host tissues' regeneration abilities with scaffolds alone or the combination of scaffolds and signaling factors (Fig. 1). Therefore, it seems advisable to start by considering simple strategies before resorting to complicated strategies using cells. We believe that in tissue engineering, using stem cells is not an end, but a means that has to be justified and optimized for individual cases. To advance this field more steadily and rapidly than ever before, we should attempt to build multi-disciplinary collaboration, which will open a new avenue for the realization of tissue-engineering-based therapies.
Figures and Tables
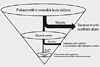 | Fig. 1Proposed flow chart of decision-making in bone tissue engineering. The majority of patients with irreversible bone defects can be treated with high-performance scaffolds alone. A minority will be treated with the combination of scaffolds and osteogenic factors to induce host tissues' regeneration abilities. The rest, representing a very small fraction, will require cell transplantation in combination with scaffolds and osteogenic factors. |
References
1. Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003. 423:332–336.
2. Tessier P, Kawamoto H, Matthews D, Posnick J, Raulo Y, Tulasne JF, Wolfe SA. Autogenous bone grafts and bone substitutes: tools and techniques: I. A 20,000-case experience in maxillofacial and craniofacial surgery. Plast Reconstr Surg. 2005. 116:5 Suppl. 6S–24S.
3. Eppley BL, Pietrzak WS, Blanton MW. Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon. J Craniofac Surg. 2005. 16:981–989.
4. Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noël D. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009. 27:307–314.
5. Hallman M, Thor A. Bone substitutes and growth factors as an alternative/complement to autogenous bone for grafting in implant dentistry. Periodontol 2000. 2008. 47:172–192.
6. Langer R, Vacanti JP. Tissue engineering. Science. 1993. 260:920–926.
7. Lanza RP, Langer RS, Vacanti J. Principles of tissue engineering. 2000. 2nd ed. San Diego: Academic Press.
8. Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002. 8:147–159.
9. St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999. 13:2072–2086.
10. Long F, Chung UI, Ohba S, McMahon J, Kronenberg HM, McMahon AP. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development. 2004. 131:1309–1318.
11. Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997. 89:755–764.
12. Komori T. Requisite roles of Runx2 and Cbfb in skeletal development. J Bone Miner Metab. 2003. 21:193–197.
13. Patel MS, Karsenty G. Regulation of bone formation and vision by LRP5. N Engl J Med. 2002. 346:1572–1574.
14. Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996. 84:911–921.
15. Ogata N, Chikazu D, Kubota N, Terauchi Y, Tobe K, Azuma Y, Ohta T, Kadowaki T, Nakamura K, Kawaguchi H. Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone turnover. J Clin Invest. 2000. 105:935–943.
16. Lee K, Chan CK, Patil N, Goodman SB. Cell therapy for bone regeneration: bench to bedside. J Biomed Mater Res B Appl Biomater. 2009. 89:252–263.
17. Bridwell KH, Anderson PA, Boden SD, Vaccaro AR, Zigler JE. What's new in spine surgery. J Bone Joint Surg Am. 2004. 86-A:1587–1596.
18. Geesink RG, Hoefnagels NH, Bulstra SK. Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J Bone Joint Surg Br. 1999. 81:710–718.
19. Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002. 84-A:1032–1044.
20. Franceschi RT, Wang D, Krebsbach PH, Rutherford RB. Gene therapy for bone formation: in vitro and in vivo osteogenic activity of an adenovirus expressing BMP7. J Cell Biochem. 2000. 78:476–486.
21. Kawaguchi H, Jingushi S, Izumi T, Fukunaga M, Matsushita T, Nakamura T, Mizuno K, Nakamura K. Local application of recombinant human fibroblast growth factor-2 on bone repair: a dose-escalation prospective trial on patients with osteotomy. J Orthop Res. 2007. 25:480–487.
22. Kawaguchi H, Oka H, Jingushi S, Izumi T, Fukunaga M, Sato K, Matsushita T, Nakamura K. TESK Group. A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: a randomized, placebo-controlled trial. J Bone Miner Res. 2010. 25:2735–2743.
23. Byers BA, Guldberg RE, García AJ. Synergy between genetic and tissue engineering: Runx2 overexpression and in vitro construct development enhance in vivo mineralization. Tissue Eng. 2004. 10:1757–1766.
24. Byers BA, Pavlath GK, Murphy TJ, Karsenty G, García AJ. Cell-type-dependent up-regulation of in vitro mineralization after overexpression of the osteoblast-specific transcription factor Runx2/Cbfal. J Bone Miner Res. 2002. 17:1931–1944.
25. Kojima H, Uemura T. Strong and rapid induction of osteoblast differentiation by Cbfa1/Til-1 overexpression for bone regeneration. J Biol Chem. 2005. 280:2944–2953.
26. Yang S, Wei D, Wang D, Phimphilai M, Krebsbach PH, Franceschi RT. In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation. J Bone Miner Res. 2003. 18:705–715.
27. Ohba S, Ikeda T, Kugimiya F, Yano F, Lichtler AC, Nakamura K, Takato T, Kawaguchi H, Chung UI. Identification of a potent combination of osteogenic genes for bone regeneration using embryonic stem (ES) cell-based sensor. FASEB J. 2007. 21:1777–1787.
28. Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999. 286:1946–1949.
29. Civitelli R. In vitro and in vivo effects of ipriflavone on bone formation and bone biomechanics. Calcif Tissue Int. 1997. 61:Suppl 1. S12–S14.
30. Notoya K, Yoshida K, Tsukuda R, Taketomi S. Effect of ipriflavone on expression of markers characteristic of the osteoblast phenotype in rat bone marrow stromal cell culture. J Bone Miner Res. 1994. 9:395–400.
31. Notoya K, Nagai H, Oda T, Gotoh M, Hoshino T, Muranishi H, Taketomi S, Sohda T, Makino H. Enhancement of osteogenesis in vitro and in vivo by a novel osteoblast differentiation promoting compound, TAK-778. J Pharmacol Exp Ther. 1999. 290:1054–1064.
32. Ohba S, Nakajima K, Komiyama Y, Kugimiya F, Igawa K, Itaka K, Moro T, Nakamura K, Kawaguchi H, Takato T, Chung UI. A novel osteogenic helioxanthin-derivative acts in a BMP-dependent manner. Biochem Biophys Res Commun. 2007. 357:854–860.
33. Zhao J, Ohba S, Shinkai M, Chung UI, Nagamune T. Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner. Biochem Biophys Res Commun. 2008. 369:444–448.
34. Hojo H, Igawa K, Ohba S, Yano F, Nakajima K, Komiyama Y, Ikeda T, Lichtler AC, Woo JT, Yonezawa T, Takato T, Chung UI. Development of high-throughput screening system for osteogenic drugs using a cell-based sensor. Biochem Biophys Res Commun. 2008. 376:375–379.
35. de Crombrugghe B, Lefebvre V, Nakashima K. Regulatory mechanisms in the pathways of cartilage and bone formation. Curr Opin Cell Biol. 2001. 13:721–727.
36. Kolettas E, Muir HI, Barrett JC, Hardingham TE. Chondrocyte phenotype and cell survival are regulated by culture conditions and by specific cytokines through the expression of Sox-9 transcription factor. Rheumatology (Oxford). 2001. 40:1146–1156.
37. Stheneur C, Dumontier MF, Guedes C, Fulchignoni-Lataud MC, Tahiri K, Karsenty G, Corvol MT. Basic fibroblast growth factor as a selective inducer of matrix Gla protein gene expression in proliferative chondrocytes. Biochem J. 2003. 369(Pt 1):63–70.
38. Church VL, Francis-West P. Wnt signalling during limb development. Int J Dev Biol. 2002. 46:927–936.
39. Zehentner BK, Dony C, Burtscher H. The transcription factor Sox9 is involved in BMP-2 signaling. J Bone Miner Res. 1999. 14:1734–1741.
40. Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, Danielson KG, Hall DJ, Tuan RS. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003. 278:41227–41236.
41. Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, Tanaka J, Bunyaratvej A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004. 320:914–919.
42. Nixon AJ, Fortier LA, Williams J, Mohammed H. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J Orthop Res. 1999. 17:475–487.
43. Hunziker EB, Rosenberg LC. Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. J Bone Joint Surg Am. 1996. 78:721–733.
44. Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002. 84:276–288.
45. Fan H, Hu Y, Qin L, Li X, Wu H, Lv R. Porous gelatin-chondroitin-hyaluronate tri-copolymer scaffold containing microspheres loaded with TGF-beta1 induces differentiation of mesenchymal stem cells in vivo for enhancing cartilage repair. J Biomed Mater Res A. 2006. 77:785–794.
46. Ishii I, Mizuta H, Sei A, Hirose J, Kudo S, Hiraki Y. Healing of full-thickness defects of the articular cartilage in rabbits using fibroblast growth factor-2 and a fibrin sealant. J Bone Joint Surg Br. 2007. 89:693–700.
47. Moore EE, Bendele AM, Thompson DL, Littau A, Waggie KS, Reardon B, Ellsworth JL. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage. 2005. 13:623–631.
48. Ikeda T, Kamekura S, Mabuchi A, Kou I, Seki S, Takato T, Nakamura K, Kawaguchi H, Ikegawa S, Chung UI. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004. 50:3561–3573.
49. Liu G, Kawaguchi H, Ogasawara T, Asawa Y, Kishimoto J, Takahashi T, Chung UI, Yamaoka H, Asato H, Nakamura K, Takato T, Hoshi K. Optimal combination of soluble factors for tissue engineering of permanent cartilage from cultured human chondrocytes. J Biol Chem. 2007. 282:20407–20415.
50. Takahashi T, Ogasawara T, Kishimoto J, Liu G, Asato H, Nakatsuka T, Uchinuma E, Nakamura K, Kawaguchi H, Chung UI, Takato T, Hoshi K. Synergistic effects of FGF-2 with insulin or IGF-I on the proliferation of human auricular chondrocytes. Cell Transplant. 2005. 14:683–693.
51. Friedenstein AJ, Piatetzky S II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966. 16:381–390.
52. Ohgushi H, Goldberg VM, Caplan AI. Heterotopic osteogenesis in porous ceramics induced by marrow cells. J Orthop Res. 1989. 7:568–578.
53. Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999. 96:10711–10716.
54. Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999. 284:1168–1170.
55. Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995. 18:1417–1426.
56. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
57. Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999. 5:309–313.
58. Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, McCarville ME, Orchard PJ, Pyeritz RE, Brenner MK. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001. 97:1227–1231.
59. Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001. 344:385–386.
60. Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002. 10:199–206.
61. Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004. 13:595–600.
62. Kuroda R, Ishida K, Matsumoto T, Akisue T, Fujioka H, Mizuno K, Ohgushi H, Wakitani S, Kurosaka M. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007. 15:226–231.
63. Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007. 1:74–79.
64. Wakitani S, Okabe T, Horibe S, Mitsuoka T, Saito M, Koyama T, Nawata M, Tensho K, Kato H, Uematsu K, Kuroda R, Kurosaka M, Yoshiya S, Hattori K, Ohgushi H. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011. 5:146–150.
65. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005. 52:2521–2529.
66. Koga H, Muneta T, Nagase T, Nimura A, Ju YJ, Mochizuki T, Sekiya I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008. 333:207–215.
67. Nimura A, Muneta T, Koga H, Mochizuki T, Suzuki K, Makino H, Umezawa A, Sekiya I. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008. 58:501–510.
68. Koga H, Engebretsen L, Brinchmann JE, Muneta T, Sekiya I. Mesenchymal stem cell-based therapy for cartilage repair: a review. Knee Surg Sports Traumatol Arthrosc. 2009. 17:1289–1297.
69. Bruder SP, Caplan AI. Lanza RP, Langer RS, Vacanti J, editors. Bone regeneration through cellular engineering. Principles of tissue engineering. 2000. 2nd ed. San Diego: Academic Press;683–696.
70. Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A. 2002. 99:4397–4402.
71. Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, Wang CY. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002. 20:587–591.
72. Bradley A. Robertson EJ, editor. Production and analysis of chimaeric mice. Teratocarcinomas and embryonic stem cells. 1987. 1st ed. Oxford: IRL Press;113–151.
73. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981. 292:154–156.
74. Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981. 78:7634–7638.
75. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998. 282:1145–1147.
76. Buttery LD, Bourne S, Xynos JD, Wood H, Hughes FJ, Hughes SP, Episkopou V, Polak JM. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 2001. 7:89–99.
77. Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997. 64:295–312.
78. Phillips BW, Belmonte N, Vernochet C, Ailhaud G, Dani C. Compactin enhances osteogenesis in murine embryonic stem cells. Biochem Biophys Res Commun. 2001. 284:478–484.
79. Kawaguchi J, Mee PJ, Smith AG. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone. 2005. 36:758–769.
80. zur Nieden NI, Kempka G, Ahr HJ. In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation. 2003. 71:18–27.
81. Warotayanont R, Zhu D, Snead ML, Zhou Y. Leucine-rich amelogenin peptide induces osteogenesis in mouse embryonic stem cells. Biochem Biophys Res Commun. 2008. 367:1–6.
82. Jukes JM, van Blitterswijk CA, de Boer J. Skeletal tissue engineering using embryonic stem cells. J Tissue Eng Regen Med. 2010. 4:165–180.
83. Jukes JM, Both SK, Leusink A, Sterk LM, van Blitterswijk CA, de Boer J. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci U S A. 2008. 105:6840–6845.
84. Kramer J, Hegert C, Guan K, Wobus AM, Müller PK, Rohwedel J. Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mech Dev. 2000. 92:193–205.
85. Sui Y, Clarke T, Khillan JS. Limb bud progenitor cells induce differentiation of pluripotent embryonic stem cells into chondrogenic lineage. Differentiation. 2003. 71:578–585.
86. Vats A, Bielby RC, Tolley N, Dickinson SC, Boccaccini AR, Hollander AP, Bishop AE, Polak JM. Chondrogenic differentiation of human embryonic stem cells: the effect of the micro-environment. Tissue Eng. 2006. 12:1687–1697.
87. Hwang NS, Varghese S, Elisseeff J. Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PLoS One. 2008. 3:e2498.
88. Tanaka H, Murphy CL, Murphy C, Kimura M, Kawai S, Polak JM. Chondrogenic differentiation of murine embryonic stem cells: effects of culture conditions and dexamethasone. J Cell Biochem. 2004. 93:454–462.
89. Oldershaw RA, Baxter MA, Lowe ET, Bates N, Grady LM, Soncin F, Brison DR, Hardingham TE, Kimber SJ. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010. 28:1187–1194.
90. Tashiro K, Inamura M, Kawabata K, Sakurai F, Yamanishi K, Hayakawa T, Mizuguchi H. Efficient adipocyte and osteoblast differentiation from mouse induced pluripotent stem cells by adenoviral transduction. Stem Cells. 2009. 27:1802–1811.
91. Kao CL, Tai LK, Chiou SH, Chen YJ, Lee KH, Chou SJ, Chang YL, Chang CM, Chen SJ, Ku HH, Li HY. Resveratrol promotes osteogenic differentiation and protects against dexamethasone damage in murine induced pluripotent stem cells. Stem Cells Dev. 2010. 19:247–258.
92. Bilousova G, Jun du H, King KB, De Langhe S, Chick WS, Torchia EC, Chow KS, Klemm DJ, Roop DR, Majka SM. Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vivo. Stem Cells. 2011. 29:206–216.
93. Ye JH, Xu YJ, Gao J, Yan SG, Zhao J, Tu Q, Zhang J, Duan XJ, Sommer CA, Mostoslavsky G, Kaplan DL, Wu YN, Zhang CP, Wang L, Chen J. Critical-size calvarial bone defects healing in a mouse model with silk scaffolds and SATB2-modified iPSCs. Biomaterials. 2011. 32:5065–5076.
94. Teramura T, Onodera Y, Mihara T, Hosoi Y, Hamanishi C, Fukuda K. Induction of mesenchymal progenitor cells with chondrogenic property from mouse-induced pluripotent stem cells. Cell Reprogram. 2010. 12:249–261.
95. Hirata K, Tsukazaki T, Kadowaki A, Furukawa K, Shibata Y, Moriishi T, Okubo Y, Bessho K, Komori T, Mizuno A, Yamaguchi A. Transplantation of skin fibroblasts expressing BMP-2 promotes bone repair more effectively than those expressing Runx2. Bone. 2003. 32:502–512.
96. Krebsbach PH, Gu K, Franceschi RT, Rutherford RB. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Hum Gene Ther. 2000. 11:1201–1210.
97. Hiramatsu K, Sasagawa S, Outani H, Nakagawa K, Yoshikawa H, Tsumaki N. Generation of hyaline cartilaginous tissue from mouse adult dermal fibroblast culture by defined factors. J Clin Invest. 2011. 121:640–657.
98. Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A. 2002. 99:12600–12605.
99. Storrie H, Stupp SI. Cellular response to zinc-containing organoapatite: an in vitro study of proliferation, alkaline phosphatase activity and biomineralization. Biomaterials. 2005. 26:5492–5499.
100. van den Dolder J, Bancroft GN, Sikavitsas VI, Spauwen PH, Jansen JA, Mikos AG. Flow perfusion culture of marrow stromal osteoblasts in titanium fiber mesh. J Biomed Mater Res A. 2003. 64:235–241.
101. Tortelli F, Cancedda R. Three-dimensional cultures of osteogenic and chondrogenic cells: a tissue engineering approach to mimic bone and cartilage in vitro. Eur Cell Mater. 2009. 17:1–14.
102. Epinette JA, Manley MT. Hydroxyapatite-coated total knee replacement: clinical experience at 10 to 15 years. J Bone Joint Surg Br. 2007. 89:34–38.
103. Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, Mastrogiacomo M, Cancedda R. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007. 13:947–955.
104. Mastrogiacomo M, Cancedda R, Quarto R. Effect of different growth factors on the chondrogenic potential of human bone marrow stromal cells. Osteoarthritis Cartilage. 2001. 9:Suppl A. S36–S40.
105. Uemura T, Dong J, Wang Y, Kojima H, Saito T, Iejima D, Kikuchi M, Tanaka J, Tateishi T. Transplantation of cultured bone cells using combinations of scaffolds and culture techniques. Biomaterials. 2003. 24:2277–2286.
106. Hatoko M, Tada H, Tanaka A, Yurugi S, Niitsuma K, Iioka H. The use of calcium phosphate cement paste for the correction of the depressed nose deformity. J Craniofac Surg. 2005. 16:327–331.
107. Tomita S, Molloy S, Jasper LE, Abe M, Belkoff SM. Biomechanical comparison of kyphoplasty with different bone cements. Spine (Phila Pa 1976). 2004. 29:1203–1207.
108. Dorozhkin SV, Epple M. Biological and medical significance of calcium phosphates. Angew Chem Int Ed Engl. 2002. 41:3130–3146.
109. Tamai N, Myoui A, Tomita T, Nakase T, Tanaka J, Ochi T, Yoshikawa H. Novel hydroxyapatite ceramics with an interconnective porous structure exhibit superior osteoconduction in vivo. J Biomed Mater Res. 2002. 59:110–117.
110. Eppley BL. Craniofacial reconstruction with computer-generated HTR patient-matched implants: use in primary bony tumor excision. J Craniofac Surg. 2002. 13:650–657.
111. Fischer-Brandies E, Dielert E. Clinical use of tricalciumphosphate and hydroxyapatite in maxillofacial surgery. J Oral Implantol. 1985. 12:40–44.
112. Karashima S, Takeuchi A, Matsuya S, Udoh K, Koyano K, Ishikawa K. Fabrication of low-crystallinity hydroxyapatite foam based on the setting reaction of alpha-tricalcium phosphate foam. J Biomed Mater Res A. 2009. 88:628–633.
113. Tada H, Hatoko M, Tanaka A, Kuwahara M, Mashiba K, Yurugi S, Iioka H, Niitsuma K. Preshaped hydroxyapatite tricalcium-phosphate implant using three-dimensional computed tomography in the reconstruction of bone deformities of craniomaxillofacial region. J Craniofac Surg. 2002. 13:287–292.
114. Igawa K, Mochizuki M, Sugimori O, Shimizu K, Yamazawa K, Kawaguchi H, Nakamura K, Takato T, Nishimura R, Suzuki S, Anzai M, Chung UI, Sasaki N. Tailor-made tricalcium phosphate bone implant directly fabricated by a three-dimensional ink-jet printer. J Artif Organs. 2006. 9:234–240.
115. Saijo H, Igawa K, Kanno Y, Mori Y, Kondo K, Shimizu K, Suzuki S, Chikazu D, Iino M, Anzai M, Sasaki N, Chung UI, Takato T. Maxillofacial reconstruction using custom-made artificial bones fabricated by inkjet printing technology. J Artif Organs. 2009. 12:200–205.
116. Saijo H, Mori Y, Fujihara H, Kanno Y, Chikazu D, Ohkubo K, Hikiji H, Iino M, Yonehara Y, Takato T. Evaluation and analysis of formation of bone at the palate in patients with cleft lip and palate after palatoplasty based on computed tomograms and three-dimensional data. Scand J Plast Reconstr Surg Hand Surg. 2010. 44:21–25.
117. Oka K, Murase T, Moritomo H, Goto A, Sugamoto K, Yoshikawa H. Corrective osteotomy using customized hydroxyapatite implants prepared by preoperative computer simulation. Int J Med Robot. 2010. 6:186–193.
118. Alvarez-Barreto JF, Shreve MC, Deangelis PL, Sikavitsas VI. Preparation of a functionally flexible, three-dimensional, biomimetic poly(L-lactic acid) scaffold with improved cell adhesion. Tissue Eng. 2007. 13:1205–1217.
119. Cuddihy MJ, Kotov NA. Poly(lactic-co-glycolic acid) bone scaffolds with inverted colloidal crystal geometry. Tissue Eng Part A. 2008. 14:1639–1649.
120. Freed LE, Vunjak-Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, Langer R. Biodegradable polymer scaffolds for tissue engineering. Biotechnology (N Y). 1994. 12:689–693.
121. Goh YQ, Ooi CP. Fabrication and characterization of porous poly(L-lactide) scaffolds using solid-liquid phase separation. J Mater Sci Mater Med. 2008. 19:2445–2452.
122. Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000. 21:2529–2543.
123. Leung L, Chan C, Baek S, Naguib H. Comparison of morphology and mechanical properties of PLGA bioscaffolds. Biomed Mater. 2008. 3:025006.
124. Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000. 21:2335–2346.
125. Tsuji H. Poly(lactide) stereocomplexes: formation, structure, properties, degradation, and applications. Macromol Biosci. 2005. 5:569–597.
126. Wang S, Cui W, Bei J. Bulk and surface modifications of polylactide. Anal Bioanal Chem. 2005. 381:547–556.
127. Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997. 36:17–28.
128. Ishaug-Riley SL, Crane-Kruger GM, Yaszemski MJ, Mikos AG. Three-dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials. 1998. 19:1405–1412.
129. Porter JR, Ruckh TT, Popat KC. Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol Prog. 2009. 25:1539–1560.
130. Nie H, Wang CH. Fabrication and characterization of PLGA/HAp composite scaffolds for delivery of BMP-2 plasmid DNA. J Control Release. 2007. 120:111–121.
131. Miyamoto S, Takaoka K, Okada T, Yoshikawa H, Hashimoto J, Suzuki S, Ono K. Evaluation of polylactic acid homopolymers as carriers for bone morphogenetic protein. Clin Orthop Relat Res. 1992. (278):274–285.
132. Miyamoto S, Takaoka K, Okada T, Yoshikawa H, Hashimoto J, Suzuki S, Ono K. Polylactic acid-polyethylene glycol block copolymer. A new biodegradable synthetic carrier for bone morphogenetic protein. Clin Orthop Relat Res. 1993. (294):333–343.
133. Saito N, Okada T, Toba S, Miyamoto S, Takaoka K. New synthetic absorbable polymers as BMP carriers: plastic properties of poly-D,L-lactic acid-polyethylene glycol block copolymers. J Biomed Mater Res. 1999. 47:104–110.
134. Saito N, Okada T, Horiuchi H, Murakami N, Takahashi J, Nawata M, Ota H, Nozaki K, Takaoka K. A biodegradable polymer as a cytokine delivery system for inducing bone formation. Nat Biotechnol. 2001. 19:332–335.
135. Kato M, Namikawa T, Terai H, Hoshino M, Miyamoto S, Takaoka K. Ectopic bone formation in mice associated with a lactic acid/dioxanone/ethylene glycol copolymer-tricalcium phosphate composite with added recombinant human bone morphogenetic protein-2. Biomaterials. 2006. 27:3927–3933.
136. Yoneda M, Terai H, Imai Y, Okada T, Nozaki K, Inoue H, Miyamoto S, Takaoka K. Repair of an intercalated long bone defect with a synthetic biodegradable bone-inducing implant. Biomaterials. 2005. 26:5145–5152.
137. Taguchi S, Namikawa T, Ieguchi M, Takaoka K. Reconstruction of bone defects using rhBMP-2-coated devitalized bone. Clin Orthop Relat Res. 2007. 461:162–169.
138. Hoshino M, Egi T, Terai H, Namikawa T, Kato M, Hashimoto Y, Takaoka K. Repair of long intercalated rib defects in dogs using recombinant human bone morphogenetic protein-2 delivered by a synthetic polymer and beta-tricalcium phosphate. J Biomed Mater Res A. 2009. 90:514–521.
139. Namikawa T, Terai H, Suzuki E, Hoshino M, Toyoda H, Nakamura H, Miyamoto S, Takahashi N, Ninomiya T, Takaoka K. Experimental spinal fusion with recombinant human bone morphogenetic protein-2 delivered by a synthetic polymer and beta-tricalcium phosphate in a rabbit model. Spine (Phila Pa 1976). 2005. 30:1717–1722.
140. Sung HJ, Meredith C, Johnson C, Galis ZS. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials. 2004. 25:5735–5742.
141. Wahl DA, Czernuszka JT. Collagen-hydroxyapatite composites for hard tissue repair. Eur Cell Mater. 2006. 11:43–56.
142. Marcacci M, Berruto M, Brocchetta D, Delcogliano A, Ghinelli D, Gobbi A, Kon E, Pederzini L, Rosa D, Sacchetti GL, Stefani G, Zanasi S. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005. (435):96–105.
143. Ochi M, Uchio Y, Tobita M, Kuriwaka M. Current concepts in tissue engineering technique for repair of cartilage defect. Artif Organs. 2001. 25:172–179.
144. Ochi M, Uchio Y, Kawasaki K, Wakitani S, Iwasa J. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Br. 2002. 84:571–578.
145. Iwasa J, Engebretsen L, Shima Y, Ochi M. Clinical application of scaffolds for cartilage tissue engineering. Knee Surg Sports Traumatol Arthrosc. 2009. 17:561–577.
146. Visna P, Pasa L, Cizmár I, Hart R, Hoch J. Treatment of deep cartilage defects of the knee using autologous chondrograft transplantation and by abrasive techniques: a randomized controlled study. Acta Chir Belg. 2004. 104:709–714.
147. Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002. 59:63–72.
148. Villanueva I, Hauschulz DS, Mejic D, Bryant SJ. Static and dynamic compressive strains influence nitric oxide production and chondrocyte bioactivity when encapsulated in PEG hydrogels of different crosslinking densities. Osteoarthritis Cartilage. 2008. 16:909–918.
149. Ossendorf C, Kaps C, Kreuz PC, Burmester GR, Sittinger M, Erggelet C. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res Ther. 2007. 9:R41.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download