Abstract
We report here on a rare case of adrenal paragonimiasis that presented with an adrenal incidentaloma. A 52-year-old male presented with fatigue and weight loss. The laboratory findings revealed eosinophilia (8.5%) and an increased eosinophil count (910/µL). The computed tomography scan showed 6.5 × 5 cm sized multilocular cystic mass in the right adrenal gland, which was non-functioning, and cystic lesions with variable sizes were also noted in the abdominal cavity. On the surgical field, whitish patches were spread out in the peritoneum, the omentum, the dome of the liver and the diaphragm. The right adrenal gland was replaced by a cystic mass filled with mucopurulent creamy materials. The pathologic findings revealed numerous eggs of Paragonimus spp. Also, the ELISA was positive for IgG paragonimus antibody. The adrenal gland can become infected by various microbial pathogens, including parasites, although it is relatively uncommon. However, in the case of a cystic adrenal mass with accompanying eosinophilia in an endemic area, clinicians should consider the possibility of parasitic infection.
Due to recent progress in the abdominal imaging techniques and its widespread use, clinicians often encounter incidentally discovered adrenal mass [1,2]. Although there are wide variations in its prevalence depending on the study population and diagnosing modality, it is generally known to be about 4-6% [3-5]. The majority of adrenal incidentalomas are benign non-functioning adenoma. From the radiologic point of view, cystic lesions are rare constituting about 1-1.9% of adrenal incidentalomas [4,6,7].
We experienced a patient presenting with cystic adrenal incidentaloma, which finally turned out to be paragonimiasis. This is an extremely rare cause of adrenal infection and therefore we report it with a review of relevant literatures.
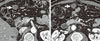
A 52-year-old male presented with fatigue and a weight loss of 5 kg over 6 months. The patient was referred for the evaluation of adrenal incidentaloma detected by ultrasonography; other malignancy check up did not show abnormality. He had past histories of type 2 diabetes and hypertension which had been treated with oral hypoglycemic agents and an antihypertensive agent for the previous 4 years. There were neither histories of other endocrine diseases nor infectious diseases. He did not drink alcohol. On admission, his vital signs were stable without fever. No symptoms and signs suggested Cushing's syndrome or pheochromocytoma. Laboratory findings revealed no abnormalities, except leukocytosis (white blood count 10,130/cm), eosinophilia (8.5%) and increased absolute eosinophil count (910/µL). In the evaluation of the functionality of adrenal mass, Cushing's syndrome was excluded by the tests for 24-hour urine free cortisol (55.9 µg/day) and 1 mg overnight dexamethasone suppression test (< 1.0 µg/dL). Primary aldosteronism was also excluded by PAC/PRA ratio (0.63). Concentrations of vanillylmandelic acid (2.87 mg/day), metanephrine (0.25 mg/day) from 24-hour urine collection excluded the possibility of pheochromocytoma. Chest X-ray was normal but computed tomography (CT) scan showed 6.5 × 5 cm sized multilocular septated cystic mass in right adrenal gland (Fig. 1A, B). Multiple, variable sized cystic lesions were also noted in the gastrocolic ligament, transverse mesocolon, and upper omentum areas (Fig. 1B, C). Although there was no hormonal overproduction, laparoscopic adrenalectomy was done due to its large size and for the confirmatory diagnosis. In the surgical field, multilobulated cystic adrenal mass was observed above the upper pole of right kidney and whitish patches were spread out in peritoneum, omentum, dome of liver and diaphragm (Fig. 2A). Right adrenal gland was totally replaced by cystic mass filled with mucopurulent creamy materials (Fig. 2B). Cross section of surgical specimen revealed variable-sized, multicystic mass (Fig. 2C). Light microscopic examination revealed chronic granulomatous inflammation with central necrosis containing numerous eggs of Paragonimus spp (Fig. 3). The eggs of Paragonimus spp were also found in peritoneum specimen. For immunologic diagnosis, ELISA was performed and the IgG titer of paragonimus antibody was positive (8.90) (Table 1).
Retrospective history taking revealed that he had ingested a raw crabs-preserved in soy sauce in Seomjin river, 2 years ago. His wife, eaten together had visited another hospital a year ago due to right-side pleural effusion and diagnosed as P. westermani infection.
He was treated with praziquantel at a total dose of 150 mg per kg of body weight divided into three doses per day for 2 days. Follow-up CT scan taken 5 months (Fig. 4) after medical treatment showed little interval change of cystic lesions in the abdominal cavity. However, the proportion of eosinophils (4.7%), eosinophil count (360/µL) and IgG titer of paragonimus antibody (2.28) were significantly decreased (Table 1).
We report here a rare case of adrenal infection, paragonimiasis. To our knowledge, there have been only 2 cases described in the literature [8,9]. In our case, the diagnosis of paragonimiasis was supported not only by pathologic findings but also by serologic testing and the history of raw crab ingestion in the endemic area. This is an unusual finding in where pulmonary parasitism was not detected.
Although adrenal incidentaloma is frequently found in the daily practice, diagnosed in up to 4-6% of examinees [3-5], cystic lesions are rarely found. In one series of 1049 incidental adrenal lesions detected by CT scan, only 13 (1%) lesions were cystic [4,6]. The causes of adrenal cysts include lymphangioma, hemangioma, cystic adenoma and infections.
While various microbial pathogens can be infected, parasitic infection of the adrenal gland rarely occurs. They can involve multiple organs concomitantly, but isolated adrenal gland infection may take place. Not only immunocompromised patients but also immunocompetent hosts can be infested. There are several case reports of hydatid disease caused by Echinococcus spp, and it is estimated to account for 6-7% of adrenal cysts [10-12]. Other causes include Leishmania spp, Trypanosoma spp, Microsporidia spp and amebic species which also can present as a cystic form [7]. However, the incidence of the adrenal paragonimiasis is extremely rare. Basically, cystic lesions caused by Paragonimus could not be resolved by drug treatment. Rather, shrinkage of mass size might be resultant from healing process with residual scar formation [13]. Treatment of adrenal paragonimiasis and pulmonary paragonimiasis is not different at all. Due to lack of cases, however, nothing much is known about the progress and prognosis of adrenal paragonimiasis, except that cystic lesion may remain in abdominal cavity.
Paragonimiasis is endemic in African and Asian countries such as China, Japan, Taiwan and Korea. The nationwide prevalence of human paragonimiasis in China was estimated to be 1.71% [14]. It is also known to be widely distributed in Korea, although the prevalence is gradually decreasing [15]. With the possibility of developing extrapulmonary paragonimiasis, involvement of brain, spinal cord, subcutaneous tissue and urinary tract have been reported in Korea [15]. However, as the infestation of organs such as omentum and adrenal gland are less likely to produce definite symptoms, diagnosis of abdominal paragonimiasis may be difficult unless remarkable lesions are found by imaging studies.
Paragonimiasis is a food-borne disease. When humans ingest inadequately cooked crustaceans such as crabs or crayfishes containing metacercariae, the metacercariae excyst in the duodenum and penetrate the intestinal wall to reach the peritoneal cavity. They migrate to the pleural cavity and lungs where they become encapsulated and develop into adult worms [16]. In this case, although the exact cause is unknown, Paragonimus spp remained only in the abdominal cavity and right adrenal gland. There was no obvious occurrence in the lungs, and therefore Paragonimus spp could not develop into adult worms. With the evidences of no lesions in the lung but ectopic lesions including adrenal gland, it can be suggested that very small number of metaceracriae were introduced into the host so that the worm lost their power to invade the lung tissue. After then the host immune cells may invade to fill the cyst. Recent studies suggest that enzymatic hydrolysis of host proteins by cysteine proteases is an essential step in host cell invasion and migration [17,18]. One hypothesis could be that there was some problem in this process which showed developmental stage-specific expression of the enzyme.
In this report, we described an extremely rare case of paragonimiasis involving adrenal gland and abdominal cavity which was incidentally discovered and verified through histologic findings. As there are no distinctive symptoms in adrenal paragonimiasis, suspicion is important. In a case of cystic adrenal mass, accompanying eosinophilia in an endemic area, it would be necessary to consider the possibility of adrenal paragonimiasis, and to conduct histological examination and immunologic tests with thorough history taking on eating habits.
Figures and Tables
 | Fig. 1Initial abdominal CT scan. A. Abdominal computed tomography scan showing 6.5 × 5 cm sized multilocular cystic mass (arrow) in the right adrenal gland. B, C. Multiple, variable sized cystic lesions (arrows) were shown in the gastrocolic ligament, transverse mesocolon, and upper omentum areas. |
 | Fig. 2Surgical findings and specimen. A. Whitish patches were disseminated in peritoneum, omentum, dome of liver and diaphragm. B, C. Adrenal gland was replaced by cystic mass filled with mucopurulent creamy materials. |
 | Fig. 3Microscopic examination revealed chronic granulomatous inflammation with central necrosis containing numerous eggs of Paragonimus spp (H&E staining, A, × 100; B, × 400). |
References
1. Young WF Jr. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007. 356:601–610.
2. Young WF Jr. Management approaches to adrenal incidentalomas. A view from Rochester, Minnesota. Endocrinol Metab Clin North Am. 2000. 29:159–185.
3. Bovio S, Cataldi A, Reimondo G, Sperone P, Novello S, Berruti A, Borasio P, Fava C, Dogliotti L, Scagliotti GV, Angeli A, Terzolo M. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006. 29:298–302.
4. Song J, Li C, Tong C, Yang H, Yang X, Zhang J, Deng Y. Evaluation of left ventricular rotation and twist using speckle tracking imaging in patients with atrial septal defect. J Huazhong Univ Sci Technolog Med Sci. 2008. 28:190–193.
5. Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev. 1995. 16:460–484.
6. Cicala MV, Sartorato P, Mantero F. Incidentally discovered masses in hypertensive patients. Best Pract Res Clin Endocrinol Metab. 2006. 20:451–466.
7. Paolo WF Jr, Nosanchuk JD. Adrenal infections. Int J Infect Dis. 2006. 10:343–353.
8. Muta K. Dawes B, editor. Paragonimus and paragonimiasis. Advances in parasitology. 1965. London and New York Academic Press Inc.
9. Hahn ST, Park SH, Kim CY, Shinn KS. Adrenal paragonimiasis simulating adrenal tumor--a case report. J Korean Med Sci. 1996. 11:275–277.
10. Toppino M, Serra GC, Bolla C, Capalbo MT, Capuzzi P, Comorio A, Mistrangelo M. Adrenal location of the echinococcus. Considerations on a clinical case. Minerva Gastroenterol Dietol. 1996. 42:221–225.
11. Akcay MN, Akcay G, Balik AA, Boyuk A. Hydatid cysts of the adrenal gland: review of nine patients. World J Surg. 2004. 28:97–99.
12. Kamishima T, Harabayashi T, Ishikawa S, Kubota KC, Nonomura K, Omatsu T, Onodera Y, Shirato H, Terae S. Alveolar hydatid disease of the adrenal gland: computed tomography and magnetic resonance imaging findings. Jpn J Radiol. 2009. 27:225–228.
13. Cho SY, Kong Y, Yun DH, Kang SY, Kim LS, Chung YB, Yang HJ. Persisting antibody reaction in paragonimiasis after praziquantel treatment is elicited mainly by egg antigens. Korean J Parasitol. 2000. 38:75–84.
14. Liu Q, Wei F, Liu W, Yang S, Zhang X. Paragonimiasis: an important food-borne zoonosis in China. Trends Parasitol. 2008. 24:318–323.
15. Choi DW. Paragonimus and paragonimiasis in Korea. Korean J Parasitol. 1990. 28:Suppl. 79–102.
16. Im JG, Kong Y, Shin YM, Yang SO, Song JG, Han MC, Kim CW, Cho SY, Ham EK. Pulmonary paragonimiasis: clinical and experimental studies. Radiographics. 1993. 13:575–586.
17. Na BK, Kim SH, Lee EG, Kim TS, Bae YA, Kang I, Yu JR, Sohn WM, Cho SY, Kong Y. Critical roles for excretory-secretory cysteine proteases during tissue invasion of Paragonimus westermani newly excysted metacercariae. Cell Microbiol. 2006. 8:1034–1046.
18. Shin MH, Kita H, Park HY, Seoh JY. Cysteine protease secreted by Paragonimus westermani attenuates effector functions of human eosinophils stimulated with immunoglobulin G. Infect Immun. 2001. 69:1599–1604.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download