Abstract
Papillary thyroid carcinoma could be a rare cause of malignant pleural effusion. The development of malignant pleural effusion in patients with papillary thyroid cancer is an extremely adverse prognostic indicator. Here, we report four cases that showed development of malignant pleural effusion during the clinical course of the papillary thyroid carcinoma and consider the prognosis. In four patients, the median survival time after the development of malignant pleural effusion was only 17 months.
Malignant pleural effusion usually occurs in patients with primary lung cancer, breast cancer or with malignant lymphoma. Median survival after diagnosis in those cancers had been reported to be from 3 to 12 months [1,2]. Metastatic papillary thyroid carcinoma (PTC) could be a rare cause of malignant pleural effusion. The overall survival rate of patients with PTC at 10 years approaches 80 to 95 percent with standard treatment [3] but, the development of malignant pleural effusion is known as an adverse prognostic indicator in those patients [4]. Although a few cases of malignant pleural effusion from PTC had been reported, neither the prognosis nor the clinical courses of them has been well described in the literature [4-10]. We report here 4 cases which showed malignant pleural effusion caused by metastatic PTC and describe their clinical courses.
A 24-year-old woman had right thyroidectomy with right neck node dissection in 1977, due to PTC with metastases to right cervical lymph nodes. After surgery, she neither underwent a follow-up examination nor thyroxine treatment.
In September 2004, she presented with left neck mass. Computed tomography (CT) of chest noted low attenuated small nodule with calcification on the left lobe of thyroid, both cervical lymphadenopathy and miliary lung nodules. Fine needle aspiration (FNA) of cervical lymph nodes revealed metastatic PTC. She subsequently had total thyroidectomy, both modified radical neck dissection (MRND) and 7.4 GBq of I-131 treatment. Post-therapeutic whole body scan (RxWBS) showed uptake by multiple lung nodules and stimulated serum thyroglobulin was 810 ng/mL. 7.4 GBq of I-131 treatment was followed 2 times additionally, but there were no improvement in size of the lung nodules.
In February 2008, 4 years after neck dissection, she came to our emergency department for worsening of shortness of breath. Chest radiography showed large amount of pleural effusion in the left hemithorax. Diagnostic thoracentesis revealed exudates with protein concentration of 4.9 g/dL, lactate dehydrogenase (LDH) concentration of 198 IU/L. The leukocyte count of fluid was 1,800/mm3 with 42% of malignant cells. Cytology showed typical papillae structures of columnar cells (Fig. 1). Thyroid function test revealed TSH 0.16 mU/L, free T4 1.4 ng/mL, thyroglobulin 34.8 ng/mL. Intercostal tube insertion and pleurodesis were performed.
Four months later, she presented with painful hemorrhagic nodule at the chest tube insertion site. The mass was considered as a metastatic nodule. Radio-frequency ablation and 7.4 GBq of I-131 treatment were followed, but she died because of the uncontrolled pleural effusion at September 2008, only 6 months after development of malignant pleural effusion.
A 64-year-old woman had total thyroidectomy and right MRND due to right neck mass confirmed as PTC in 1999. Surgical and pathological findings showed 3 cm mass in right thyroid which invaded perithyroidal soft tissue with right cervical lymph nodes metastasis. 5.6 GBq of I-131 treatment was followed with uptake in thyroid bed.
In August 2000, 11 years after initial diagnosis, stimulated serum thyroglobulin was 33.7 ng/mL but, I-131 diagnostic whole body scan (DxWBS) showed no abnormal uptake. Tc-99m MIBI SPECT was revealed hypermetabolic lesions at left supraclavicular area. She underwent FNA of lymph nodes and it turned out to be metastatic PTC. Left MRND was performed in December 2000.
Three years later, she presented with left neck mass again. Neck ultrasonography (USG) and whole body fluorodeoxyglucose-positron emission tomography scan showed multiple cervical lymphadenopathy and lung nodules. FNA of enlarged cervical lymph node noted metastatic PTC. She had radiation therapy of cervical area (23 fractions, 4,600 cGy) and thorax area (10 fractions, 1,800 cGy). No evidence of residual disease was noted in neck but, serum thyroglobulin level was still high (3.0-4.7 ng/mL, TSH 1.6 mU/L).
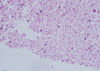
In July 2008, 4 years after radiation therapy, she sought medical attention for progressive dyspnea. Chest radiography revealed large amount of right pleural effusion. Serum thyroglobulin was 10.0 ng/mL (TSH 1.7 mU/L). Pleural fluid was exudates (Protein 5.2 g/dL, LDH 191 IU/L) and the leukocyte count of fluid was 300/mm3 including 37% of malignant cells. But, cytology of fluid could not diagnose the origin of cancer cells (Fig. 2). To evaluate the cause of pleural effusion, pleural fluid thyroglobulin was checked. It was 115 ng/mL and high enough to diagnose pleural effusion as malignant effusion from thyroid. Chest tube insertion was performed and pleurodesis with doxycycline 800 mg was followed.
Four months later, chest radiography showed increased amount of right pleural effusion. 5.6 GBq of I-131 treatment was performed but, there was no uptake. She died in August 2009, 1 year after diagnosis of malignant pleural effusion.
A 68-year-old man had routine health checkup at May 2002 and neck USG detected 3.5 cm thyroid nodule. FNA of thyroid nodule showed typical papillary structure. He had total thyroidectomy and pathology revealed 5.5 cm thyroid mass that metastasize to the paratracheal lymph nodes. 5.6 GBq of I-131 treatment was followed.
RxWBS noted multifocal increased uptakes of iodine in abdominal cavity, right chest wall, thus CT of chest and abdomen were performed. Multiple variable sized nodules at basal lungs with 1.3 cm nodule at left adrenal gland were detected and considered as metastatic PTC. 7.4 GBq I-131 therapy was done twice consecutively but, RxWBS showed increased number and size of nodules. After two more 7.4 GBq I-131 treatments, RxWBS revealed no definite uptakes. However, chest radiography couldn't show improvement.
In December 2008, 6 years after initial diagnosis, chest radiography detected right pleural effusion. It was hemorrhagic pleural fluid with protein concentration 4.8 g/dL, LDH 455 IU/L and the total leukocyte count of pleural fluid was 1,200/mm3. Cytologic analyses showed papillary structure of atypical cells and pleural fluid thyroglobulin was significantly high as 1,610 ng/mL. He was diagnosed as malignant pleural effusion caused by metastasis of PTC. Intercostal tube insertion and pleurodesis with doxycycline 1,000 mg were also done.
CT of chest showed worsening of lung metastasis with newly developed multiple pleural nodules which suspected as metastatic PTC (Fig. 3). One large pleural nodule was close to thoracic vertebrae body, so he underwent prophylactic radiation therapy to pleural nodules (15 fractions, 4,500 cGy).
After that, brain, kidney, liver and multiple bone metastases are combined. He underwent palliative radiotherapy to metastatic bone lesions for relieving pain. He died in September 2010, 2 years after development of malignant pleural effusion.
A 55-year-old man presented with right supraclavicular mass in 1998. He diagnosed as PTC by FNA of thyroid. Total thyroidectomy and bilateral MRND were performed but, complete resection of thyroid cancer was failed because of severe perithyroidal tissue adhesion. Pathology noted a follicular variant type of PTC involving cervical lymph nodes. 5.6 GBq I-131 treatment was followed. At that time, stimulated serum thyroglobulin was 406 ng/mL and RxWBS showed diffuse increased activity in both lung fields.
Three months later, DxWBS was performed. It showed no definite abnormal uptakes but, serum thyroglobulin was still high as 400 ng/mL. 5.6 GBq I-131 therapy was performed additionally and RxWBS also revealed no definite uptakes.
Routine chest radiography in May 2001, 12 years after initial diagnosis, noted multiple diffuse lung nodules. Stimulated serum thyroglobulin was 1,380 mg/mL and CT of chest revealed multiple metastatic lung nodules with right cervical lymphadenopathy. FNA of cervical lymph nodes documented metastatic PTC. Cervical lymph node dissection was followed.
Two years later, metastatic lung nodules deteriorated further and left pleural effusion was detected. 7.4 GBq of I-131 therapy was planned diagnostic thoracentesis was performed ahead. Pleural fluid was exudates with the total leukocyte count 1,700/mm3 including 29% of malignant cells. Cytologic examination showed papillary proliferation of cells with psammoma bodies. Chest tube insertion with pleurodesis was followed. RxWBS showed multiple hypermetabolic nodules in diffuse lung fields, pleura and mediastinum.
Systemic chemotherapy (Tegafur-Uracil/vinorelbine) was administered 3 times but no response was documented and he presented with mass at the chest tube insertion site in February 2004. Palliative radiotherapy and additional 7.4 GBq I-131 therapy was done but, he finally died in January 2005, about 2 years after diagnosis of malignant pleural effusion.
PTC is generally known as having a favorable prognosis. But, some undergo frequent recurrence and eventually die from disease progression. It is important to identify the high risk patients early and treat correctly [8].
In this report, 3 patients (patient 2, 3, and 4 in Table 1) were over 50 years and had cervical lymphadenopathy, extrathyroidal invasion at the time of initial surgery. Moreover, 2 patients among them (patient 3 and 4 in Table 1) had distant metastatic lesions at the time of initial diagnosis. They were considered as high risk group and had aggressive treatment with total thyroidectomy and radioiodine ablation therapy. But, the disease showed recurrence and progression. In patient 1 (Table 1), she was young at the time of diagnosis and disease was stable over 20 years. The metastatic progression appeared late but, disease progression after the appearance of pleural effusion was extremely rapid.
In retrospective report of Vassilopoulous-sellin and Sneige [8], only 10 patients (0.6%) had malignant pleural effusion during the course of PTC among 1,772 patients. Regardless of the timing of the development of pleural effusion, the disease exhibited aggressive biologic behavior with rapid deterioration and its appearance carries an adverse prognostic significance. The median overall survival time was 27 months after the diagnosis of thyroid cancer and 11 months after the development of pleural effusion [8]. At this report, the median overall survival time after the development of malignant pleural effusion of 4 patients was also only 17 months (Table 2).
In reported cases so far, malignant pleural effusion resulting from metastasis of PTC had been diagnosed when cytologic examination show papillary structures of epithelial cells in pleural fluid with or without psammoma bodies [9]. In patient 3 (Table 1), cytology noted atypical papillary clusters and pleural fluid thyroglobulin was also high as 1,610 ng/mL (serum thyroglobulin 171 ng/mL). In patient 2 (Table 1), cytologic examination of pleural fluid could not identify the primary cancer foci due to degeneration. We checked thyroglobulin level in pleural fluid. It was 115 ng/mL which was significantly higher compared with serum thyroglobulin level (10.0 ng/mL). We could diagnose the pleural effusion due to metastatic differentiated thyroid carcinoma based on pleural fluid thyroglobulin level. It would be the first reported case that pleural fluid of unknown cause diagnosed as malignant pleural effusion due to differentiated thyroid carcinoma by using pleural fluid thyroglobulin level.
During the course of the disease and before the diagnosis of pleural effusion, radiologically apparent lung metastasis were found in all cases in this report as well as the report of Vassilopoulous-sellin and Sneige [8]. However, Jung et al. [6] reported a patient presented with malignant pleural effusion and pleural nodules without lung metastasis and Jeong et al. [5] reported malignant pleural effusion associated with breast metastasis.
Radioiodine therapy, local radiotherapy and systemic chemotherapy could be applied for the treatment of malignant pleural effusion from PTC but, nothing was proved to be effective to control malignancy itself [8]. Intercostal tube drainage with intrapleural instillation of sclerosant was preferred to control symptom, prevent recurrence of effusion and recurrent aspiration [2]. In this review, all 4 patients were treated with chest tube insertion and pleurodesis. However, patient 1 and 4 was suffered from metastatic nodule at chest tube insertion site and pleural effusion was recurred in several months in patient 1 and 2.
In conclusion, patients with PTC generally have an excellent prognosis but, high risk patients could undergo frequent recurrence and finally die of disease progression. The development of malignant pleural effusion from PTC means poor prognosis and patients with malignant pleural effusion may survive only for several months.
We report 4 cases that showed development of malignant pleural effusion during the clinical course of the PTC. Pleural fluid thyroglobulin is a useful diagnostic marker when cytologic examination could not identify the origin of malignant pleural effusion. There were no effective ways to control malignant pleural effusion and the median survival time after the development of malignant pleural effusion of 4 patients was only 17 months.
Figures and Tables
Fig. 1
Pleural fluid cytology of patient 1, which shows papillary structure of columnar cells (× 400).
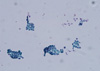
Fig. 3
Computed tomography of chest (patient 3). A. Large amount of right pleural fluid, multiple pleural and lung nodules were noted. B. There was 4 cm right paravertebral mass with internal hemorrhage and it was also regarded as pleural metastatic nodule. The patient underwent prophylactic radiation therapy to the mass.

References
1. Hausheer FH, Yarbro JW. Diagnosis and treatment of malignant pleural effusion. Cancer Metastasis Rev. 1987. 6:23–40.
2. Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ. BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010. 65:ii32–ii40.
3. Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998. 338:297–306.
4. Kim JY, Park DW, Na JO, Hwang BY, Kim DL, Shin DH, Kim SG, Choi KM, Baik SH, Choi DS, Cho SJ, Kim NH. A case of malignant pleural effusion with pleural metastasis in a patient with papillary thyroid carcinoma. J Korean Soc Endocrinol. 2002. 17:269–274.
5. Jeong J, Shin SY, Son MK, Lee YJ, Kim SH, Kie JH, Choi YJ, Hong YK, Hahn CH, Lee SM, Kim CJ. A case of pleural metastasis from papillary thyroid carcinoma. Tuberc Respir Dis. 2007. 63:188–193.
6. Jung KH, Seo JA, Lee JH, Jo WM, Kim JH, Shin C. A case of papillary thyroid cancer presenting as pleural effusion. Tuberc Respir Dis. 2008. 64:314–317.
7. Siddaraju N, Viswanathan VK, Saka VK, Basu D, Shanmugham C. Fine needle aspiration of follicular variant of papillary thyroid carcinoma presenting with pleural effusion: a case report. Acta Cytol. 2007. 51:911–915.
8. Vassilopoulou-Sellin R, Sneige N. Pleural effusion in patients with differentiated papillary thyroid cancer. South Med J. 1994. 87:1111–1116.
9. Vernon AN, Sheeler LR, Biscotti CV, Stoller JK. Pleural effusion resulting from metastatic papillary carcinoma of the thyroid. Chest. 1992. 101:1448–1450.
10. Cuervo Pinna MA, Magro Ledesma D, Arrebola García JD. Metastatic pleural effusion secondary to papillary carcinoma of the thyroid. Arch Bronconeumol. 1998. 34:566–567.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download