Abstract
Central pontine myelinolysis (CPM) by complicating rapid correction of severe hyponatremia has been widely reported. Additionally, CPM was occasionally reported among patients with post-liver transplantation, burns, chronic renal failure with dialysis, or other diseases associated with or not associated with other electrolyte changes or hyperosmolarity. However, there have been a few reports of CPM occurring in diabetic patients without documented electrolyte changes. This report is, to the best of our knowledge, the first report of CPM in type 2 diabetic patients without electrolyte changes in Korea. A 40-year-old man with type 2 diabetes mellitus with abruptly developed dysarthria and ataxia was admitted to our facility. He suffered from poor glucose control and multiple diabetic complications. Brain magnetic resonance imaging (MRI) revealed a well-defined bilateral symmetric hyperintense lesion in the central portion of the pons on T2- and diffusion-weighted images, which was consistent with CPM. After the patient's blood glucose and blood pressure normalized, his dysarthria and ataxia improved. Six months after discharge, follow-up MRI showed a persistent, but greatly reduced symmetric lesion in the central pons. It is certainly possible for CPM to be overlooked clinically in diabetic patients, but more cases could be diagnosed if careful attention was paid to this syndrome.
Central pontine myelinolysis (CPM) is a disease showing noninflammatory demyelination in the central portion of the pons, firstly described by Adams et al. [1]. CPM by complicating rapid correction of severe hyponatremia patients has been widely reported. Alcoholism or malnutrition is the most frequently associated conditions with CPM [2-4]. CPM has also been reported among the patients with post-liver transplantation, burns, chronic renal failure with dialysis, or other diseases, that was related with or without other electrolyte changes or hyperosmolarity [4-11].
Since the pontine lesions reported in diabetic patients without documented electrolyte changes are exclusive case reports of several patients, these conditions are thought to be rare [12,13]. Two cases of CPM as an unusual complication of diabetes were briefly reported a decade ago, and four diabetic patients with CPM on magnetic resonance imaging (MRI) were recently reported [12,13]. This would be the first report of CPM in type 2 diabetic patients without electrolyte changes in Korea.
Forty-year-old man with a history of type 2 diabetes and hypertension for 4 years visited and was admitted to our hospital on February 2, 2010, presenting with sudden onset dysarthria and ataxia for 10 days. He had a history of alcohol consumption of 25 g per day and a 20 pack-year history of cigarette smoking.
He was previously admitted to our hospital due to blurred vision in November 2008, at which time, diabetic neuropathy, nephropathy, and retinopathy was diagnosed. The diagnosis of diabetic neuropathy was based on decreased vibration sense and slow nerve conduction velocity of lower extremities. Ten g monofilament test was normal. Proliferative diabetic retinopathy of both eyes was observed and left eye was on almost blindness state. He received vitrectomy of right eye while admitting. He had overt proteinuria and slight elevated serum creatinine (1.4 mg/dL, normal range, 0.4-1.3). Hemoglobin A1c level was 10.9% (normal range, 4.4-6.4). He also had a 0.3 cm sized open wound and callus formation on plantar surface of big toe of left foot. Ankle brachial index was normal. He had been lost at follow-up visit since two months after discharge, and he did not get any treatment in other clinic.
On admission, his blood pressure was 160/90 mmHg and neurological tests revealed preserved ankle jerks, sensation and motor power in all extremities, but mild ataxia while walking. The patient had an alert mentality and comprehended all commands, but had mild dysarthria.
Laboratory data showed high white blood cell count (12,000/µL, normal range, 4000-10,000), normal serum blood urea nitrogen (BUN 23.2 mg/dL, normal range, 7-27) and raised serum creatinine (1.6 mg/dL, normal range, 0.4-1.3). 24 hours urine protein was over 5 g. His serum glucose level was 452 mg/dL; Hemoglobin A1c level was at a high value of 13.2% (normal range, 4.4-6.4); Serum sodium level was 135 mM (normal range, 137-150) and the corrected serum sodium level was 141 mM; Serum potassium level was 4.3 mM/L (normal range, 3.5-5.3); Serum osmolarity was 315 mOsm/kg H2O (normal range, 289-302).
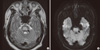
Brain MRI was taken on day 2 and revealed the well-defined legion in the central portion of pons showing bilateral symmetric hyhyperintensity on T2 and hypointensity on T1-weighted images. Diffusion-weighted images showed bilateral symmetric hyperintensity in the lesion of pons. There was only a thin rim of normal signal around the lesion at the periphery of pons, sparing the tegmentum of pons and ventrolateral pons, which was consistent with CPM (Fig. 1).
As insulin treatment, oral hypoglycemic agent, blood pressure lowering agents and other supportive care were provided, the patient's blood glucose levels and blood pressure decreased to near normal range; his dysarthria and ataxia gradually improved. He was discharged on hospital day 16.
Two months after discharge, his symptoms were almost disappeared, but he still complained about mild dysarthria during rapid speech.
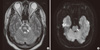
Six months after discharge, follow-up brain MRI was performed. This revealed still symmetric hyperintensity in the central portion of pons on T2-weighted and diffusion-weighted images, but the size and intensity of the hypointense area was markedly diminished (Fig. 2).
CPM is a clinicoradiologic disease, which shows noninflammatory demyelination in the central portion of the pons. It was first described in 1959 by Adams and colleagues [1] in four alcoholic or malnourished patients. Two decades after the first report, a link between these disorders and the rapid correction of hyponatremia was established [2-4]. Though it is well-known that CPM is commonly associated with the rapid correction or overcorrection of hyponatremia, other electrolyte changes such as hypernatremia and hypokalemia, and/or extreme hyperosmolarity have been identified as important factors in its pathogenesis [4-6].
Alcoholism and malnutrition is the first described and the most frequently associated conditions with CPM [1]. However, it has also been reported in patients with a variety of disease states and after certain surgical procedures. CPM was occasionally reported among the patients with burn, often associated with hypernatremia; the patients with post-liver transplantation; and the patients with chronic renal failure with dialysis [5-8].
There are a few reports of CPM occurring in diabetic patients without documented electrolyte changes [12,13]. About a decade ago, Casey et al. [12] briefly reported two cases of CPM as an unusual complication of diabetes. Recently, Ichikawa et al. [13] reported four diabetic patients with CPM on MRI, and two of them had subclinical pontine lesions. One of six patients was a type 1 diabetic patient, and three of the six patients were under dialysis treatment from chronic renal failure due to diabetic nephropathy [12,13]. Status of blood glucose control was diverse, and most patients had multiple diabetic complications and uncontrolled hypertension [12,13].
In Korea, CPM was reported in a few patients with post-liver transplantation, chronic renal failure with dialysis and acute lymphoblastic leukemia after hematopoietic stem cell transplantation [9-11]. There was a case report of extrapontine myelinolysis associated with hyperosmolar hyperglycemic syndrome and hypernatremia [14]. To the best knowledge of us, there was no report of CPM in diabetes without electrolyte changes in Korea.
Casey et al. [12] and Ichikawa et al. [13] suggested the possibilities of asymptomatic or subclinical pontine lesion before it became clinically overt and that such lesions might have been overlooked. Poorly controlled diabetic patients often develop renal dysfunction and greater osmolar shifts from elevated serum BUN and glucose levels might induce brain edema [8]; the present case showed elevated serum osmolarity on admission. Moreover, diabetes mellitus often accompanies hypertension and both diabetes mellitus and hypertension cause disruption of cerebral autoregulation, endothelial cells and the blood-brain barrier, which eventually leads to plasma leakage [15,16]. Also diabetic autonomic neuropathy commonly appears in diabetic patients and develops to orthostatic hypotension, which may cause abrupt fluctuation of blood pressure. From this, we speculated the fact that hyperosmolarity in relation with chronically poor metabolic control and multiple diabetic complications possibly lead to the disruption of endothelial cells and the blood-brain barrier that might cause demyelination [13].
The typical MRI findings show symmetric hypointensity on T1-weighted images and symmetric hyperintensity on T2-weighted and diffusion-weighted images in the central pons with sparing of the corticospinal tracts and the ventrolateral tracts [17,18].
In the original report of CPM with alcoholism and malnutrition complicating rapid correction of severe hyponatremia, the patient has usually gone through a biphasic clinical course, initially presenting with seizures from hyponatremia [1]. The second phase revealed dysarthria, dysphagia, a flaccid quadriparesis which later becomes spastic, from involvement of the basis pontis. If the lesion extends into the tegmentum of the pons pupillary, oculomotor abnormalities may occur. If lesions of extrapontine myelinolysis are also present, the clinical picture may be very confusing, a variety of apparently psychiatric and behavioural changes and movement disorders may occur. The clinical manifestations of 6 patients of CPM cases in diabetic patients without electrolyte changes vary considerably, from those of asymptomatic patient (2 patients) to the typical clinical symptoms, including diplopia, left-sided pain, marked ataxia and altered levels of consciousness [12,13].
There is no specific treatment for CPM. After the insult of CPM, management should be supportive and be directed towards minimizing morbidity and mortality. Case reports or very small series have tended to reinforce the perception that this condition has an excessively high mortality, because before computed tomography (CT)/MRI this was a post-mortem diagnosis. But, introduction of CT and MRI allowed for confident diagnosis in life and reports of asymptomatic cases. In series of 34 cases showed that only two died, 10 survived but left dependent, 11 had some deficits but were independent, 11 recovered completely [18]. The complete recovery from this condition is possible; however, maximum recovery may require several months and residual focal signal abnormalities may persist as sequelae of CPM [19]. In terms of prognosis and severity, the size of the pontine lesion does not correlate with the severity of the neurologic illness or the final outcome [19]. Most patients of CPM cases in diabetic patients without electrolyte changes showed complete recovery without sequelae within several months, and follow-up MRI in available patients revealed marked decreased size of lesion of central pons, as in our case [12,13].
We experienced and reported a case of CPM in a 40-year-old man with type 2 diabetic patient suffering from poor glucose control and multiple diabetic complications, but without documented electrolyte changes. We suggested that hyperosmolarity from chronically poor metabolic control and multiple diabetic complications, especially renal dysfunction and autonomic neuropathy, and accompanying hypertension might be important factors triggering demyelination. It is possible that this syndrome is clinically underrecognized in type 2 diabetic patient and we could diagnose more unsuspected cases if we pay attention to this syndrome. Clinicians should be aware of the possibility of CPM when treating diabetic patients with neurologic disorders, even if serum sodium or osmolarity has not changed. MRI is one of the useful tools in documenting this disease.
Figures and Tables
References
1. Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry. 1959. 81:154–172.
2. Norenberg MD, Leslie KO, Robertson AS. Association between rise in serum sodium and central pontine myelinolysis. Ann Neurol. 1982. 11:128–135.
3. Laureno R. Central pontine myelinolysis following rapid correction of hyponatremia. Ann Neurol. 1983. 13:232–242.
4. Messert B, Orrison WW, Hawkins MJ, Quaglieri CE. Central pontine myelinolysis. Considerations on etiology, diagnosis, and treatment. Neurology. 1979. 29:147–160.
5. McKee AC, Winkelman MD, Banker BQ. Central pontine myelinolysis in severely burned patients: relationship to serum hyperosmolality. Neurology. 1988. 38:1211–1217.
6. McComb RD, Pfeiffer RF, Casey JH, Wolcott G, Till DJ. Lateral pontine and extrapontine myelinolysis associated with hypernatremia and hyperglycemia. Clin Neuropathol. 1989. 8:284–288.
7. Bonham CA, Dominguez EA, Fukui MB, Paterson DL, Pankey GA, Wagener MM, Fung JJ, Singh N. Central nervous system lesions in liver transplant recipients: prospective assessment of indications for biopsy and implications for management. Transplantation. 1998. 66:1596–1604.
8. Tarhan NC, Agildere AM, Benli US, Ozdemir FN, Aytekin C, Can U. Osmotic demyelination syndrome in end-stage renal disease after recent hemodialysis: MRI of the brain. AJR Am J Roentgenol. 2004. 182:809–816.
9. Jeong HK, Gwak MS, Kim GS. Central pontine myelinolysis after liver transplantation: a case report. Korean J Anesthesiol. 2006. 50:469–473.
10. Jang SW, Kim IY, Kim JH, Song SH, Lee DW, Lee SB, Lee SJ, Kwak IS. A case of central pontine and extrapontine myelinolysis in a patient with maintenance hemodialysis. Korean J Nephrol. 2006. 25:327–331.
11. Lim KH, Kim S, Lee YS, Kim KH, Kim J, Rhee J, Kim HJ, Yi HG, Oh SY, Lim JH, Han SW, Lee S, Kim I, Yoon SS, Park S, Kim BK. Central pontine myelinolysis in a patient with acute lymphoblastic leukemia after hematopoietic stem cell transplantation: a case report. J Korean Med Sci. 2008. 23:324–327.
12. Casey E, Evans A, Krentz A, Watkins P, Hopkins D. Central pontine myelinolysis. An unusual complication of diabetes. Diabetes Care. 1999. 22:998–1000.
13. Ichikawa H, Murakami H, Katoh H, Hieda S, Kawamura M. Central pontine lesions observed with MRI in four diabetic patients. Intern Med. 2008. 47:1425–1430.
14. Koh CO, Yoon HS, Kim HK, Kim DM, Yoon DY, Lee JH, Kim WK. A case of extrapontine myelinolysis associated with hyperosmolar hyperglycemic syndrome. Korean J Med. 2005. 68:320–324.
15. Skinhoj E, Strandgaard S. Pathogenesis of hypertensive encephalopathy. Lancet. 1973. 1:461–462.
16. Iwata A, Koike F, Arasaki K, Tamaki M. Blood brain barrier destruction in hyperglycemic chorea in a patient with poorly controlled diabetes. J Neurol Sci. 1999. 163:90–93.
17. Miller GM, Baker HL Jr, Okazaki H, Whisnant JP. Central pontine myelinolysis and its imitators: MR findings. Radiology. 1988. 168:795–802.
18. Menger H, Jorg J. Outcome of central pontine and extrapontine myelinolysis (n = 44). J Neurol. 1999. 246:700–705.
19. Laubenberger J, Schneider B, Ansorge O, Gotz F, Haussinger D, Volk B, Langer M. Central pontine myelinolysis: clinical presentation and radiologic findings. Eur Radiol. 1996. 6:177–183.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download