초록
Background
The gonadotropin releasing hormone (GnRH) neurons perform a pivotal function in the central regulation of fertility. Somatostatin (SST) is an important neuromodulatory peptide in the central nervous system and alters neuronal activities via G protein-coupled SST receptors. A number of studies have shown that SST modulates the reproductive axis at the hypothalamic level. However, the precise action mechanisms of SST and related receptor subtypes have yet to be fully understood. In this study, we evaluated the direct effects of SST on GnRH neurons in juvenile mice.
Methods
Juvenile (postnatal days, < PND 30) GnRH-GFP transgenic mice expressing green fluorescent protein were used in this study. Acute coronal brain slices containing the preoptic area were prepared and all identified GnRH neurons were recorded using the gramicidin perforated-patch clamp technique; type II SST receptor (SSTR2) mRNA expression was evaluated via single cell reverse transcription-polymerase chain reaction (RT-PCR).
Results
SST caused membrane hyperpolarization, depolarization, no response, or membrane hyperpolarization with a reduction of action potential. Most (57.7%, 30/52) of the GnRH neurons tested were hyperpolarized by SST and this SST-induced hyperpolarization was found to be concentration-dependent. The percentage of responses, membrane potential changes (MPC), and resting membrane potential (RMP) by SST were not significantly different in juvenile male and female GnRH neurons. The SST-induced hyperpolarization was maintained in the presence of tetrodotoxin (TTX), a sodium channel blocker, and an amino acid blocking cocktail (AABC) containing AP-5 (NMDA receptor antagonist), CNQX (non-NMDA glutamate receptor antagonist), picrotoxin (GABAA receptor antagonist), and strychnine (glycine receptor antagonist). SSTR2 mRNA was expressed on 10 (38%) among 26 GnRH neurons. Seglitide, an SSTR2 agonist, mimicked this SST-induced hyperpolarization (11/23 47.8%) and this response was maintained in the presence of TTX and AABC.
REFERENCES
1. Herbison AE. GnRH neuron. Henry H, Norman A, editors. Encyclopedia of hormones. p. 171–177. San Diego: Academic Press;2003.
2. Yu WH, Kimura M, McCann SM. Effect of somatostatin on the release of gonadotropins in male rats. Proc Soc Exp Biol Med. 214:83–86. 1997.

3. Starcevic V, Milosevic V, Brkic B, Severs WB. Somatostatin affects morphol-ogy and secretion of pituitary luteinizing hormone (LH) cells in male rats. Life Sci. 70:3019–3027. 2002.

4. Prelevic GM, Wurzburger MI, Balint-Peric L, Nesic JS. Inhibitory effect of sandostatin on secretion of luteinising hormone and ovarian steroids in polycystic ovary syndrome. Lancet. 336:900–903. 1990.

5. Pillon D, Caraty A, Fabre-Nys C, Lomet D, Cateau M, Bruneau G. Regulation by estradiol of hypothalamic somatostatin gene expression: possible involvement of somatostatin in the control of luteinizing hormone secretion in the ewe. Biol Reprod. 71:38–44. 2004.

6. Van Vugt HH, Swarts HJ, Van de Heijning BJ, Van der Beek EM. Cen-trally applied somatostatin inhibits the estrogen-induced luteinizing hormone surge via hypothalamic gonadotropin-releasing hormone cell activation in female rats. Biol Reprod. 71:813–819. 2004.
7. Herbison AE. Multimodal influence of estrogen upon gonadotropin-releas-ing hormone neurons. Endocr Rev. 19:302–330. 1998.

8. Flanagan-Cato LM. Estrogen-induced remodeling of hypothalamic neural circuitry. Front Neuroendocrinol. 21:309–329. 2000.

9. Robinson JE, Birch RA, Grindrod JA, Taylor JA, Unsworth WP. Sexually differentiated regulation of gnRH release by gonadal steroid hormones in sheep. Reprod Suppl. 61:299–310. 2003.

10. Bhattarai JP, Kaszas A, Park SA, Yin H, Park SJ, Herbison AE, Han SK, Abraham IM. Somatostatin inhibition of gonadotropin-releasing hormone neurons in female and male mice. Endocrinology. 151:3258–3266. 2010.

11. Rozen S, Skaletsky H. Primer3 on the WWW for general users and for bi-ologist programmers. Misener S, Krawetz S, editors. Bioinformatics methods and protocols: methods in molecular biology. p. 365–386. Totowa: Humana Press;2000.

12. Rhee JS, Ebihara S, Akaike N. Gramicidin perforated patch-clamp tech-nique reveals glycine-gated outward chloride current in dissociated nucleus solitarii neurons of the rat. J Neurophysiol. 72:1103–1108. 1994.

13. Han SK, Herbison AE. Norepinephrine suppresses gonadotropin-releasing hormone neuron excitability in the adult mouse. Endocrinology. 149:1129–1135. 2008.

14. Mollenholt P, Rawal N, Gordh T Jr, Olsson Y. Intrathecal and epidural somatostatin for patients with cancer. Analgesic effects and postmortem neuropathologic investigations of spinal cord and nerve roots. Anesthesiol-ogy. 81:534–542. 1994.
15. Jiang N, Furue H, Katafuchi T, Yoshimura M. Somatostatin directly inhibits substantia gelatinosa neurons in adult rat spinal dorsal horn in vitro. Neu-rosci Res. 47:97–107. 2003.

16. Takeda M, Kadoi J, Takahashi M, Nasu M, Matsumoto S. Somatostatin inhibits the excitability of rat small-diameter trigeminal ganglion neurons that innervate nasal mucosa and project to the upper cervical dorsal horn via activation of somatostatin 2a receptor. Neuroscience. 148:744–756. 2007.

17. Pittman QJ, Siggins GR. Somatostatin hyperpolarizes hippocampal pyra-midal cells in vitro. Brain Res. 221:402–408. 1981.

18. Moore SD, Madamba SG, Joels M, Siggins GR. Somatostatin augments the M-current in hippocampal neurons. Science. 239:278–280. 1988.

19. Yin H, Lee KE, Park SA, Bhattarai JP, Suh BJ, Jeon JG, Kim BG, Park SJ, Han SK. Inhibitory effects of somatostatin on the substantia gelatinosa neurons of trigeminal subnucleus caudalis via somatostatin type 2 receptors in juvenile mice. Brain Res. 1304:49–56. 2009.

20. Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem. 273:18677–18680. 1998.
21. Bohm SK, Grady EF, Bunnett NW. Regulatory mechanisms that modu-late signalling by G-protein-coupled receptors. Biochem J. 322(Pt 1):1–18. 1997.
22. Premont RT, Inglese J, Lefkowitz RJ. Protein kinases that phosphorylate activated G protein-coupled receptors. FASEB J. 9:175–182. 1995.

23. Horie K, Insel PA. Retrovirally mediated transfer of a G protein-coupled receptor kinase (GRK) dominant-negative mutant enhances endogenous calcitonin receptor signaling in Chinese hamster ovary cells. GRK inhibition enhances expression of receptors and receptor mRNA. J Biol Chem. 275:29433–29440. 2000.
24. Yoon SH, Jin W, Spencer RJ, Loh HH, Thayer SA. Desensitization of del-ta-opioid-induced mobilization of Ca2+ stores in NG108-15 cells. Brain Res. 802:9–18. 1998.
25. Kramer HK, Simon EJ. Role of protein kinase C (PKC) in agonist-induced mu-opioid receptor down-regulation: I. PKC translocation to the membrane of SH-SY5Y neuroblastoma cells is induced by mu-opioid agonists. J Neurochem. 72:585–593. 1999.
26. Rapacciuolo A, Suvarna S, Barki-Harrington L, Luttrell LM, Cong M, Lefkowitz RJ, Rockman HA. Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates beta-1 adrenergic receptor endocy-tosis through different pathways. J Biol Chem. 278:35403–35411. 2003.
27. Holliday ND, Tough IR, Cox HM. A functional comparison of recombi-nant and native somatostatin sst2 receptor variants in epithelia. Br J Pharmacol. 152:132–140. 2007.

28. Young Shim E, Jung Kim H, Kim MJ, Rhie DJ, Jo YH, Kim MS, June Hahn S, Lee MY, Yoon SH. Desensitization of somatostatin-induced inhibition of low extracellular magnesium concentration-induced calcium spikes in cultured rat hippocampal neurons. Brain Res. 1111:61–71. 2006.

29. Wang HL, Dichter M, Reisine T. Lack of cross-desensitization of somatosta-tin-14 and somatostatin-28 receptors coupled to potassium channels in rat neocortical neurons. Mol Pharmacol. 38:357–361. 1990.
30. Hoyer D, Bell GI, Berelowitz M, Epelbaum J, Feniuk W, Humphrey PP, O'Carroll AM, Patel YC, Schonbrunn A, Taylor JE, Reisine T. Classifica-tion and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 16:86–88. 1995.

32. Schonbrunn A, Gu YZ, Dournard P, Beaudet A, Tannenbaum GS, Brown PJ. Somatostatin receptor subtypes: specific expression and signaling prop-erties. Metabolism. 45:8–11. 1996.

33. Schwanzel-Fukuda M, Pfaff DW. The migration of luteinizing hormone-releasing hormone (LHRH) neurons from the medial olfactory placode into the medial basal forebrain. Experientia. 46:956–962. 1990.
Fig. 1.
Effects of somatostatin (SST) on juvenile gonadotropin releasing hormone (GnRH) neurons in mice at gramicidin perforated current clamp mode. Representative traces showing membrane hyperpolarization (A. PND18, RMP = -72 mV), membrane depolarization (B. PND28, RMP = -63 mV), no response (C. PND28, RMP = -55 mV) and surcease of action potential followed by membrane hyper-polarization (D. PND14, RMP = -62 mV) by bath application of 300 nM SST. E. Stag col-umn showing percentage response by SST (H, hyperpolarization; N, no response; D, depolarization).
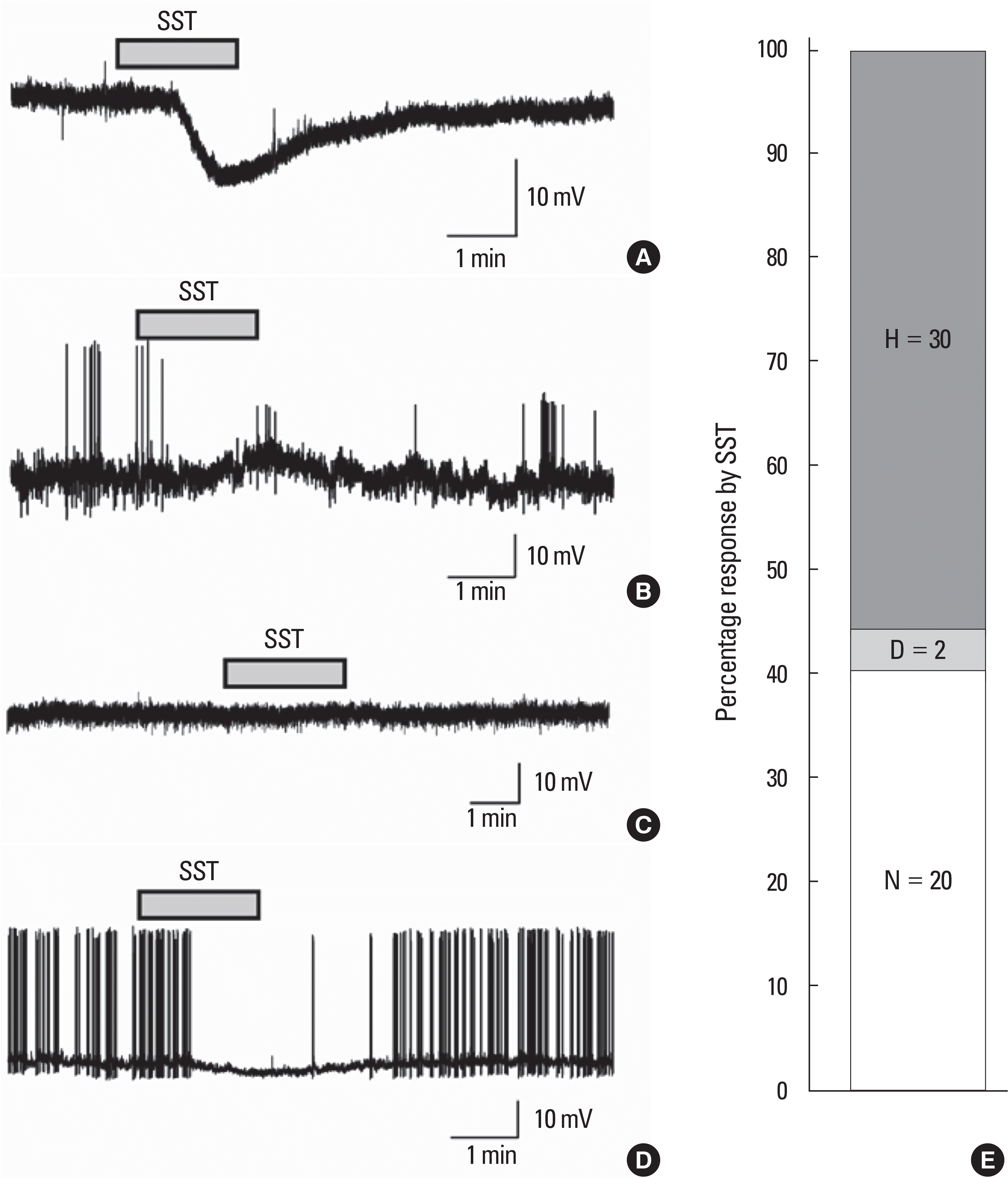
Fig. 2.
Concentration-dependent hyperpolarizing effect of SST on juvenile GnRH neurons. A. Representative trace showing concentration dependent membrane hyperpolarization by application of 10, 30, 100, and 300 nM SST (PND28, RMP = -62.0 mV). B. Concentration-response relationship. ∗represents P < 0.05.
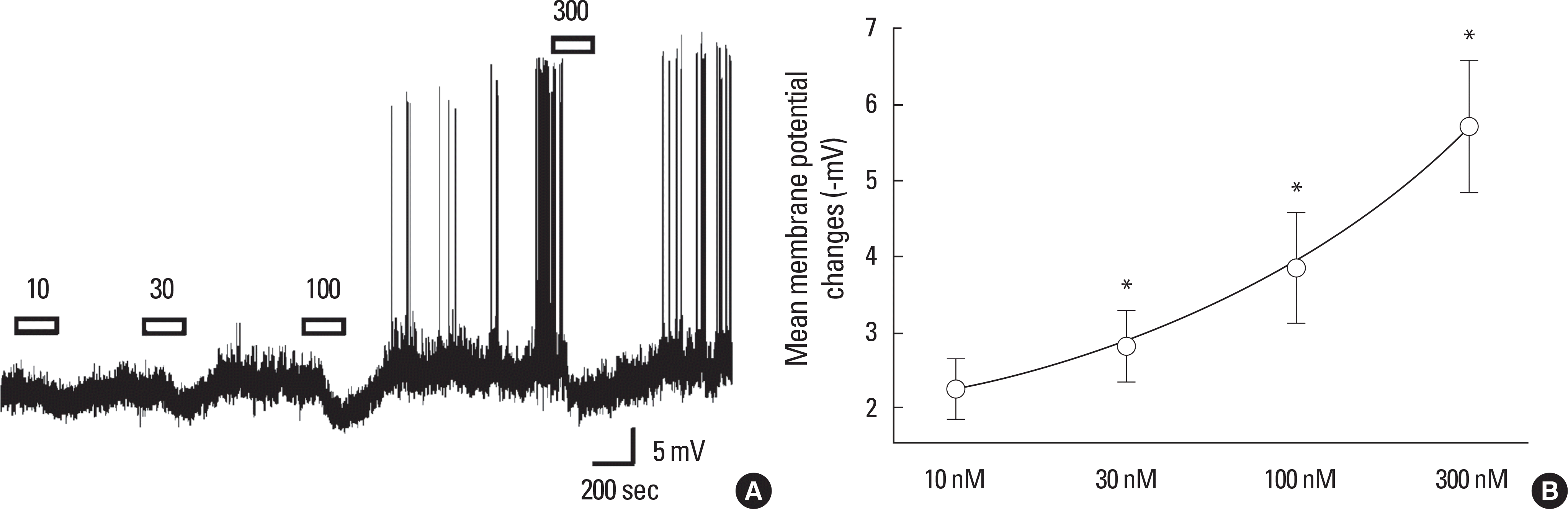
Fig. 3.
A. Percentage response by 300 nM SST in juvenile males and females. R, responded; NR, not responded. B. Comparison of the mean membrane potential changes between males and females by 300 nM SST. Found no significant difference (P > 0.05; one way ANOVA).
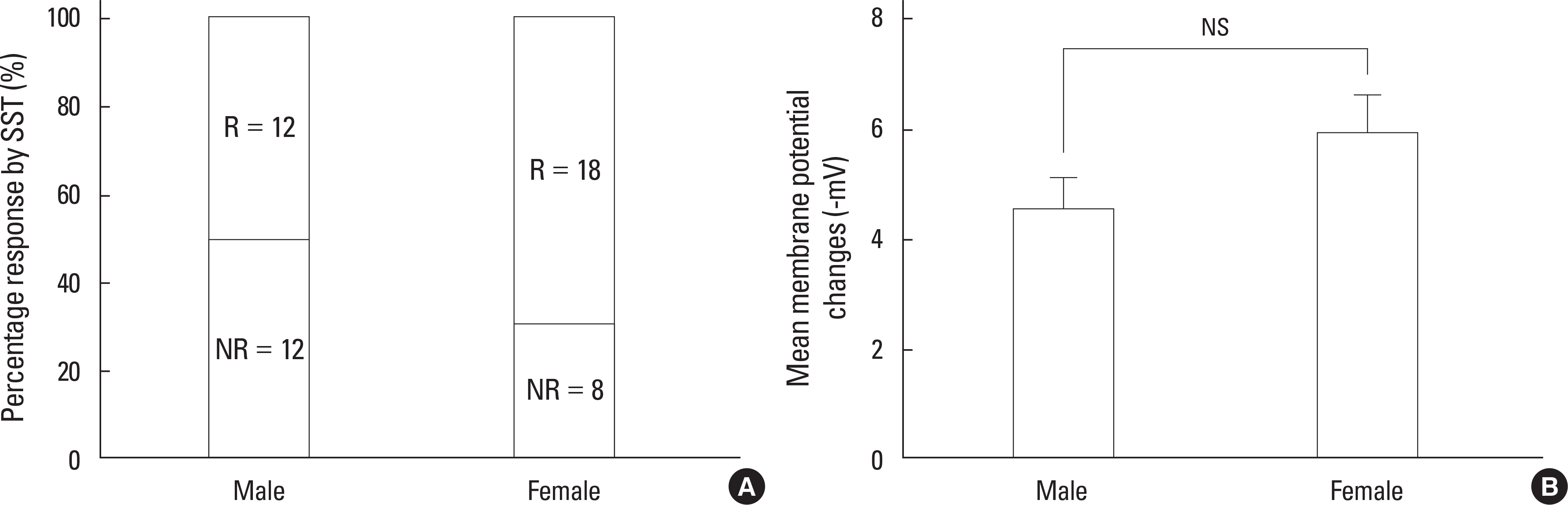
Fig. 4.
SST acts on the postsynaptic GnRH neurons directly. A. A representative trace showing hyperpolarization by successive application of 300 nM SST (PND27, RMP = -57.3 mV). Break bar depicts a time interval of 15-20 minutes. B. A representative trace showing hyperpolarization by SST and re-mains persisted in the presence of amino acid blocking cocktail (AABC) including AP-5 (20 µM, an NMDA receptor antagonist), CNQX (10 µM, a non-NMDA glutamate receptor antagonist), picrotoxin (50 µM, a GABA A receptor antag-onist), and strychnine (2 µM, a glycine receptor antagonist) with TTX (0.5 µM, a voltage-gated Na+ channel blocker) in a juvenile GnRH neuron resting at -59.6 mV. PND29. C. Relative potential changes of the 2nd response and response in the presence of AABC. ∗ and NS represent P < 0.05 and not significant, respectively (one sample t-test).
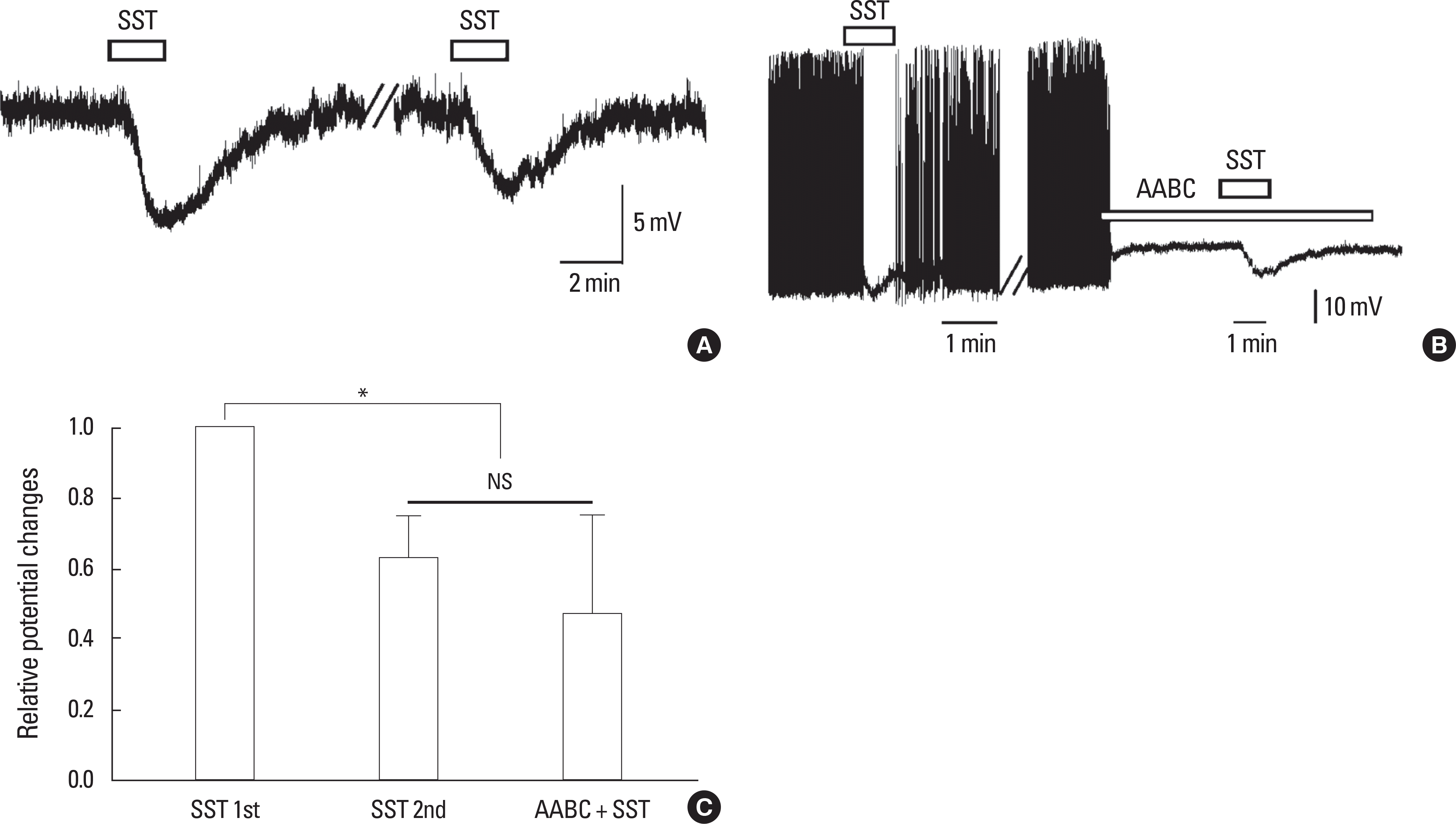
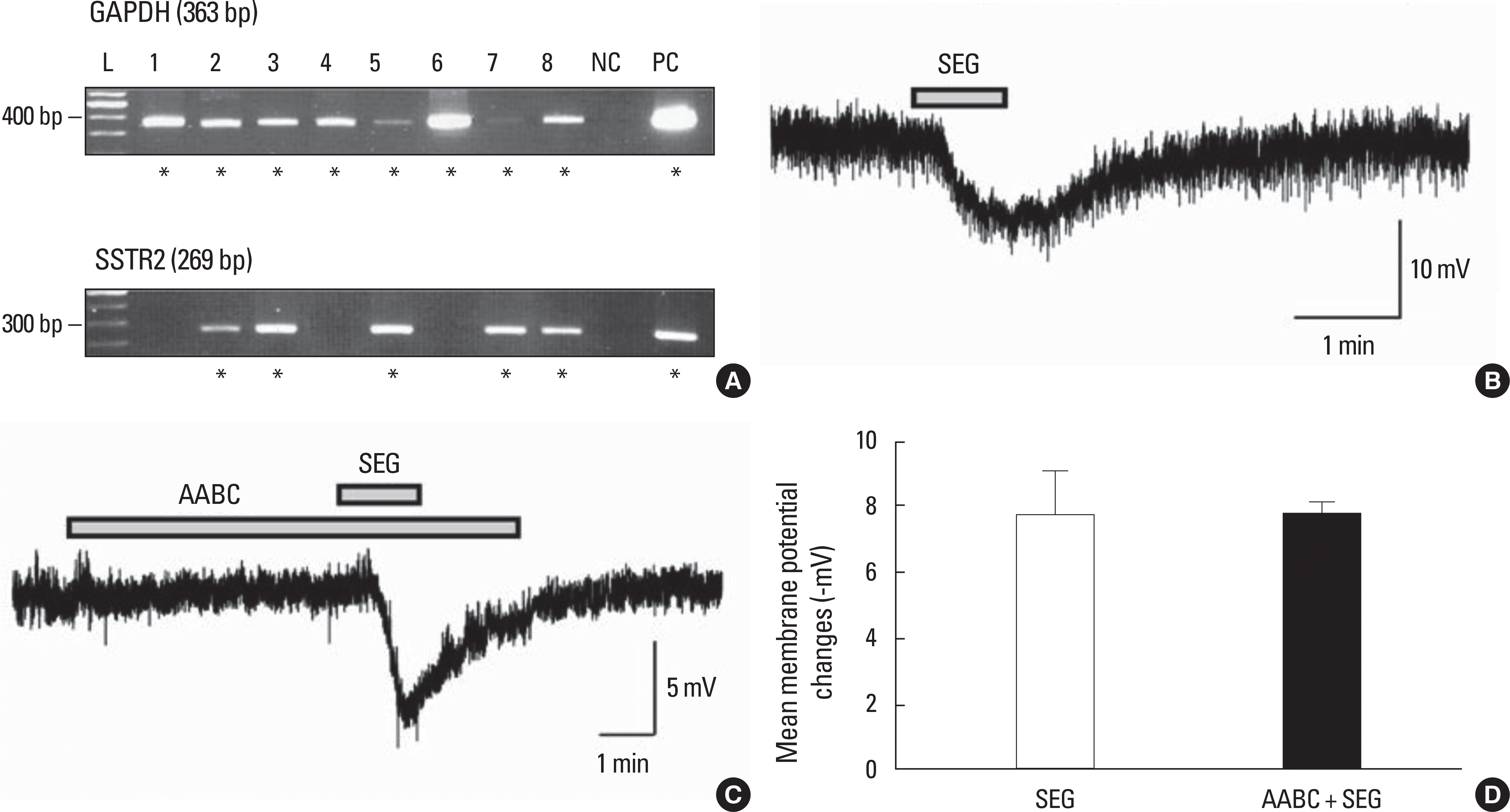
Fig. 5.
A. An example of SSTR2 mRNA expression on GnRH neurons. GAPDH, a housekeeping gene was used to conform the harvested GnRH neurons (n = 8). L (100 bp ladder), 1-8 (harvested GnRH neurons), NC (negative control, harvested GnRH neurons reacted without reverse transcriptase), PC (positive control, cDNA of total brain extract). B. A representative trace showing hyperpolarization by seglitide (SEG) (1 µM), a SSTR2 agonist from a GnRH neuron resting at -70 mV depicting mimicry of SST-induced membrane hyperpolarization (PND28). C. A representative traces showing hyperpolarization by SEG in the presence of AABC. PND13, RMP = -72 mV. D. Bar graph showing the mean membrane hyperpolarization to SEG (1 µM) and with AABC (P > 0.05).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download