초록
Thyrotropin (TSH)-secreting pituitary adenoma is a very rare disease. In one-quarter of patients suffering from this disease, the pituitary tumor secretes other anterior pituitary hormones. Herein, we report a case of pituitary adenoma with simultaneous secretion of TSH and growth hormone (GH). A 34-year-old female visitied local hospital complaining of sweating, intermittent palpita-tion, and weight loss of 8 kg within 1 year. The patient had undergone trans-sphenoidal surgery 3 years prior for resolution of a TSH and GH co-secreting pituitary adenoma. She had been administered somatostatin analogue prior to visiting our hospital. The patient's GH levels were suppressed to below 1 ng/mL on the 75 g oral glucose tolerance test, and her basal insulin-like growth factor-I (IGF-I) level was within normal range. Thyroid function tests demonstrated increased levels of both free thyroxine and TSH. Sella-MRI revealed pituitary adenoma at the floor of the pituitary fossa, approximately 2 cm in height. Therefore, she was diagnosed with residual TSH-secreting pituitary adenoma. The patient again underwent trans-sphenoidal surgery and entered complete remission, based on hormone levels and MRI findings.
Go to : 
REFERENCES
1. Beck-Peccoz P, Brucker-Davis F, Persani L, Smallridge RC, Weintraub BD. Thyrotropin-secreting pituitary tumors. Endocr Rev. 17:610–638. 1996.

2. Beck-Peccoz P, Persani L, Mannavola D, Campi I. Pituitary tumours: TSH-secreting adenomas. Best Pract Res Clin Endocrinol Metab. 23:597–606. 2009.
3. Jailer JW, Holub DA. Remission of Graves' disease following radiotherapy of a pituitary neoplasm. Am J Med. 28:497–500. 1960.

4. Swearingen B, Biller BMK. Diagnosis and management of pituitary disorders. 1st ed.p. 237–270. Totowa, NJ: Humana Press Inc;2008.
5. Beck-Peccoz P, Persani L. Thyrotropin-secreting pituitary adenomas. www.thyroidmanager.org/. (Date accessed May 13. 2011.
6. Hamilton CR Jr, Adams LC, Maloof F. Hyperthyroidism due to thyrotro-pin-producing pituitary chromophobe adenoma. N Engl J Med. 283:1077–1080. 1970.

7. Sanno N, Teramoto A, Osamura RY. Thyrotropin-secreting pituitary adenomas. Clinical and biological heterogeneity and current treatment. J Neu-rooncol. 54:179–186. 2001.
8. Beck-Peccoz P, Persani L, Mantovani S, Cortelazzi D, Asteria C. Thyrotro-pin-secreting pituitary adenomas. Metabolism. 45:75–79. 1996.

9. Beckers A, Abs R, Mahler C, Vandalem JL, Pirens G, Hennen G, Steven-aert A. Thyrotropin-secreting pituitary adenomas: report of seven cases. J Clin Endocrinol Metab. 72:477–483. 1991.

10. Kim CH, Kim GS, Kim HK, Park JY, Shong YK, Hong SB, Ko JM, Kim CJ. Pituitary thyrotropin-secreting tumors in Korean. J Korean Soc Endocrinol. 12:165–175. 1997.
11. Cohen LE, Radovick S. Molecular basis of combined pituitary hormone deficiencies. Endocr Rev. 23:431–442. 2002.

12. Mantovani G, Asteria C, Pellegrini C, Bosari S, Alberti L, Bondioni S, Pever-elli E, Spada A, Beck-Peccoz P. HESX1 expression in human normal pitu-itaries and pituitary adenomas. Mol Cell Endocrinol. 247:135–139. 2006.

13. Brucker-Davis F, Oldfield EH, Skarulis MC, Doppman JL, Weintraub BD. Thyrotropin-secreting pituitary tumors: diagnostic criteria, thyroid hormone sensitivity, and treatment outcome in 25 patients followed at the National Institutes of Health. J Clin Endocrinol Metab. 84:476–486. 1999.

14. Kronenberg H, Williams RH. Williams textbook of endocrinology. 11th ed.p. 396–397. Philadelphia, Edinburgh: Saunders Elsevier;2008.
15. Mixson AJ, Friedman TC, Katz DA, Feuerstein IM, Taubenberger JK, Col-andrea JM, Doppman JL, Oldfield EH, Weintraub BD. Thyrotropin-se-creting pituitary carcinoma. J Clin Endocrinol Metab. 76:529–533. 1993.

16. Socin HV, Chanson P, Delemer B, Tabarin A, Rohmer V, Mockel J, Steve-naert A, Beckers A. The changing spectrum of TSH-secreting pituitary adenomas: diagnosis and management in 43 patients. Eur J Endocrinol. 148:433–442. 2003.

17. Ezzat S, Horvath E, Kovacs K, Smyth HS, Singer W, Asa SL. Basic fibro-blast growth factor expression by two prolactin and thyrotropin-producing pituitary adenomas. Endocr Pathol. 6:125–134. 1995.

18. Webster J, Peters JR, John R, Smith J, Chan V, Hall R, Scanlon MF. Pituitary stone: two cases of densely calcified thyrotrophin-secreting pituitary adenomas. Clin Endocrinol (Oxf). 40:137–143. 1994.

Go to : 
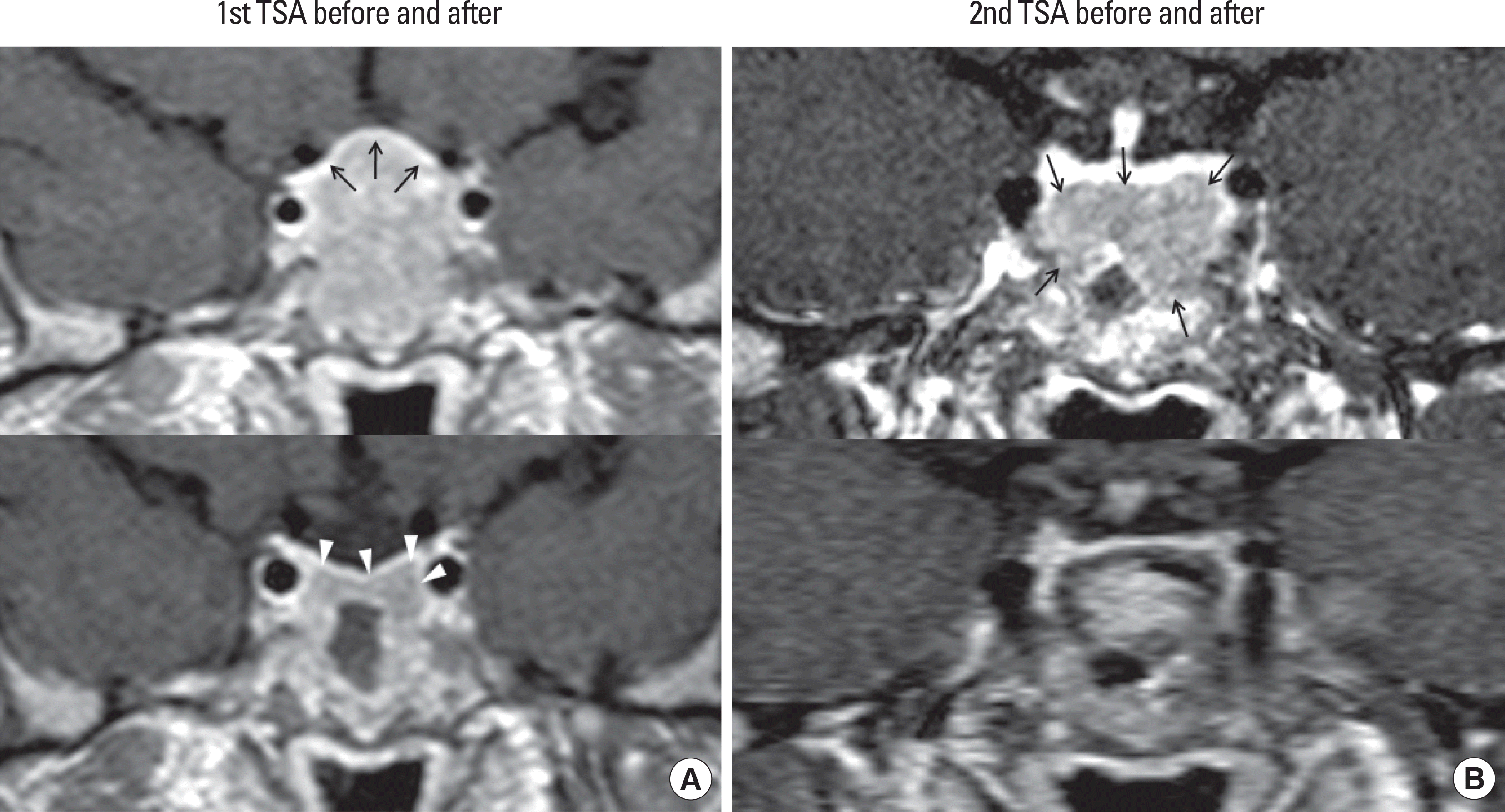 | Fig. 1.A coronal view of TSH-secreting macroadenoma by MRI, which was taken before and after the surgery. A. About 30 mm sized macroadenoma displacing the enhanced pituitary gland cranially (arrows) before the first TSA (upper panel) and residual tumor (arrow heads) after the first TSA (lower panel). B. About 20 mm sized macroadenoma (arrows) at the floor of pituitary fossa before the second TSA (upper panel) and completely resected tumor after the second the TSA (lower panel). TSA, trans-sphenoidal surgery; TSH, thyroid stimulating hormone. |
 | Fig. 2.A high-power view of the pituitary adenoma. A. Tumor tissues show regular thin fibrous septa surrounding tumor cells and chromophobe adenoma (H&E stain, × 200). B-D. Immunohistochemical staining of the tumor cells was positive to TSH (B). and GH (C). and negative to PRL (D). (× 100). GH, growth hormone; RPL, prolactin; TSH, thyroid stimulating hormone. |
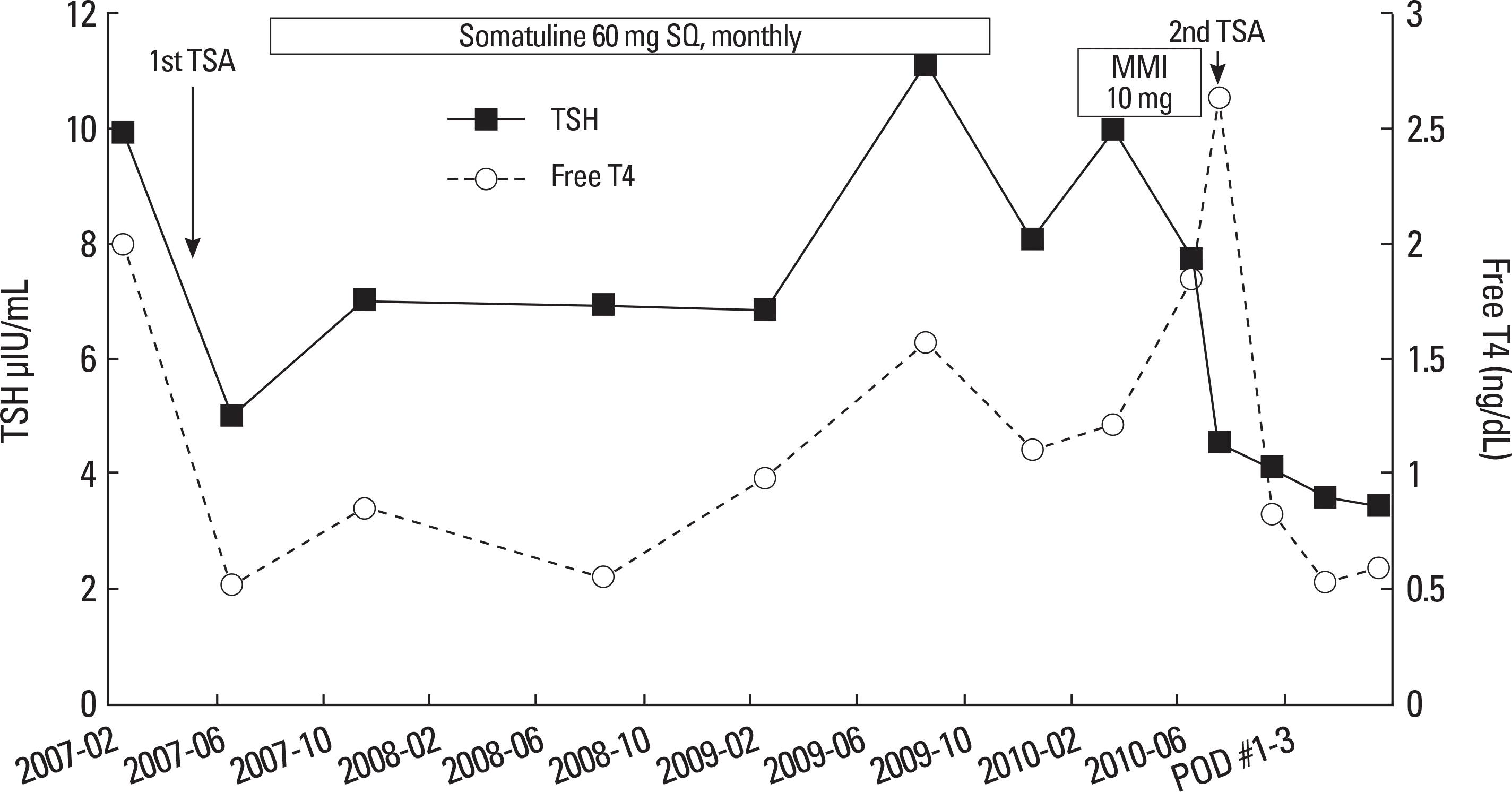 | Fig. 3.Clinical course of the patient (changes in free T4 and TSH levels). MMI, methima-zole; POD, postoperative days; T4, thyroxine; TSA, trans-sphenoidal surgery; TSH, thyroid stimulating hormone |
Table 1.
Results of 75 g glucose-GH suppression test
| Basal | 60 min | 120 min | |
|---|---|---|---|
| Glucose (mg/dL) | 82 | 193 | 160 |
| GH (ng/mL) | 2.66 | 0.63 | 0.58 |
Table 2.
Result of combined pituitary stimulation test (Regular insulin 0.1 U/kg, TRH 400 µ g, LHRH 100 µ g IV)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download