Figures and Tables
Fig. 1
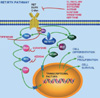
Targeted therapies that inhibit the receptor tyrosine kinase pathway. Adopted from Target Oncol 4:275-285, 2009 [1].
References
1. Capdevila J, Perez-Garcia J, Obiols G, Tabernero J. Targeted therapies in thyroid cancer. Target Oncol. 2009. 4:275–285.
2. Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, Mandel SJ, Flaherty KT, Loevner LA, O'Dwyer PJ, Brose MS. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008. 26:4714–4719.
3. Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, Liang J, Wakely PE Jr, Vasko VV, Saji M, Rittenberry J, Wei L, Arbogast D, Collamore M, Wright JJ, Grever M, Shah MH. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009. 27:1675–1684.
4. Rosen LS, Kurzrock R, Mulay M, Van Vugt A, Purdom M, Ng C, Silverman J, Koutsoukos A, Sun YN, Bass MB, Xu RY, Polverino A, Wiezorek JS, Chang DD, Benjamin R, Herbst RS. Safety, pharmacokinetics, and efficacy of AMG 706, an oral multikinase inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007. 25:2340–2342.
5. Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, Licitra L, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Schlumberger MJ. Motesanib Thyroid Cancer Study Group. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008. 359:31–42.
6. Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, Tortorici M, Shalinsky DR, Liau KF, Cohen RB. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008. 26:4708–4713.
7. Ravaud A, de la Fouchardière C, Asselineau J, Delord JP, Cao CD, Niccoli P, Rodien P, Klein M, Catargi B. Efficacy of sunitinib in advanced medullary thyroid carcinoma: intermediate results of phase II THYSU. Oncologist. 2010. 15:212–213.
8. Wells SA Jr, Gosnel JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010. 28:767–772.
9. Pennell NA, Daniels GH, Haddad RI, Ross DS, Evans T, Wirth LJ, Fidias PH, Temel JS, Gurubhagavatula S, Heist RS, Clark JR, Lynch DJ. A phase II study of gefitinib in patients with advanced thyroid cancer. Thyroid. 2008. 18:317–323.
10. Zhang Y, Guessous F, Kofman A, Schiff D, Abounader R. XL-184, a MET, VEGFR-2 and RET kinase inhibitor for the treatment of thyroid cancer, glioblastoma multiforme and NSCLC. IDrugs. 2010. 13:112–121.
11. Ain KB, Lee C, Williams KD. Phase II trial of thalidomide for therapy of radioiodine-unresponsive and rapidly progressive thyroid carcinomas. Thyroid. 2007. 17:663–670.
12. Ain KB, Lee C, Holbrook KM, Dziba JM, Williams KD. Phase II study of lenalidomide in distantly metastatic, rapidly progressive, and radioiodine-unresponsive thyroid carcinomas: preliminary results. J Clin Oncol. 2008. 26:S6027.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download