Abstract
Background
Non-functioning pituitary adenomas (NFPAs) are characterized by the absence of clinical and biochemical evidence of pituitary hormone hypersecretion, and these tumors constitute approximately one third of all the tumors of the anterior pituitary. Recently, hormonal deficiencies have gradually evolved to become the leading presenting signs and symptoms in patients with NF-PAs. We investigated pituitary hormonal insufficiencies according to the magnetic resonance imaging (MRI) findings in patients with NFPA.
Methods
We evaluated the patients who were newly diagnosed with NFPA from 1997 through 2009. Among them, we analyzed 387 patients who were tested for their combined pituitary function and who underwent MRI. The severity of the hypopituitarism was determined by the number of deficient axes of the pituitary hormones. On the MRI study, the maximal diameter of the tumor, Hardy's classification, the thickness of the pituitary gland and the presence of stalk compression were evaluated.
Results
The mean age was 46.85 ± 12.93 years (range: 15–86) and 186 patients (48.1%) were male. As assessed on MRI, the tumor diameter was 27.87 ± 9.93 mm, the thickness of the normal pituitary gland was 1.42 ± 2.07 mm and stalk compression was observed in 201 patients (51.9%). Hypopituitarism was observed in 333 patients (86.0%). Deficiency for each pituitary hormone was most severe in the patients with Hardy type IIIA. Hypopituitarism was severe in the older age patients (P = 0.001) and the patients with a bigger tumor size (P < 0.001) and the presence of stalk compression (P < 0.001). However, the patients who had a thicker pituitary gland showed less severe hypopituitarism (P < 0.001). Multivariate analysis showed that age, tumor diameter and the thickness of pituitary gland were important determinants for pituitary deficiency (P = 0.004, P < 0.001, P = 0.022, respectively).
Go to : 
References
2. Cheol Ryong Ku, Eun Jig Lee. Treatment and prognosis of nonfunctioning pituitary adenomas. Asia-Pacific Journal of Endocrinology, Epub,. 2010.
3. Cury ML, Fernandes JC, Machado HR, Elias LL, Moreira AC, Castro M. Non-functioning pituitary adenomas: clinical feature, laboratorial and imaging assessment, therapeutic management and outcome. Arq Bras Endocrinol Metabol. 53:31–39. 2009.

4. Milker-Zabel S, Debus J, Thilmann C, Schlegel W, Wannenmacher M. Fractionated stereotactically guided radiotherapy and radiosurgery in the treatment of functional and nonfunctional adenomas of the pituitary gland. Int J Radiat Oncol Biol Phys. 50:1279–1286. 2001.

5. Nomikos P, Ladar C, Fahlbusch R, Buchfelder M. Impact of primary surgery on pituitary function in patients with non-functioning pituitary ade-nomas – a study on 721 patients. Acta Neurochir (Wien). 146:27–35. 2004.

6. Lee EJ, Ahn JY, Noh T, Kim SH, Kim TS, Kim SH. Tumor tissue identification in the pseudocapsule of pituitary adenoma: should the pseudocapsule be removed for total resection of pituitary adenoma? Neurosurgery. 64:62–69. 2009.

7. Melmed S, Kleinberg D. Anterior pituitary. In: Kronenberg H, Williams RH eds. Williams textbook of endocrinology. 11th ed. pp.240–242. Philadelphia, Elsevier Saunders,. 2008.
8. Melmed S, Jameson JL. Disorders of the anterior pituitary and hypothalamus. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J eds. Harrison's principles of internal medicine. 17th ed. pp.2195–2216. New York, McGraw-Hill Medical,. 2008.
9. Mohr G, Hardy J, Comtois R, Beauregard H. Surgical management of giant pituitary adenomas. Can J Neurol Sci. 17:62–66. 1990.

11. Kassam AB, Gardner PA, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM. Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg. 108:715–728. 2008.

12. Kassam AB, Prevedello DM, Thomas A, Gardner P, Mintz A, Snyderman C, Carrau R. Endoscopic endonasal pituitary transposition for a transdor-sum sellae approach to the interpeduncular cistern. Neurosurgery. 62:57–72. 2008.

13. Kato K, Saeki N, Yamaura A. Morphological changes on MR imaging of the normal pituitary gland related to age and sex: main emphasis on pu-bescent females. J Clin Neurosci. 9:53–56. 2002.

14. Arafah BM, Kailani SH, Nekl KE, Gold RS, Selman WR. Immediate recovery of pituitary function after transsphenoidal resection of pituitary macroadenomas. J Clin Endocrinol Metab. 79:348–354. 1994.

15. Arafah BM. Reversible hypopituitarism in patients with large nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 62:1173–1179. 1986.

16. Arafah BM, Prunty D, Ybarra J, Hlavin ML, Selman WR. The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas. J Clin Endocrinol Metab. 85:1789–1793. 2000.

17. Wichers-Rother M, Hoven S, Kristof RA, Bliesener N, Stoffel-Wagner B. Non-functioning pituitary adenomas: endocrinological and clinical outcome after transsphenoidal and transcranial surgery. Exp Clin Endocrinol Diabetes. 112:323–327. 2004s.

18. Dekkers OM, Pereira AM, Roelfsema F, Voormolen JH, Neelis KJ, Schroi-jen MA, Smit JW, Romijn JA. Observation alone after transsphenoidal surgery for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab. 91:1796–1801. 2006.

19. Dekkers OM, Pereira AM, Romijn JA. Treatment and follow-up of clinically nonfunctioning pituitary macroadenomas. J Clin Endocrinol Metab. 93:3717–3726. 2008.

20. Karavitaki N, Thanabalasingham G, Shore HC, Trifanescu R, Ansorge O, Meston N, Turner HE, Wass JA. Do the limits of serum prolactin in dis-connection hyperprolactinaemia need re-definition? A study of 226 patients with histologically verified non-functioning pituitary macroadenoma. Clin Endocrinol (Oxf). 65:524–529. 2006.

Go to : 
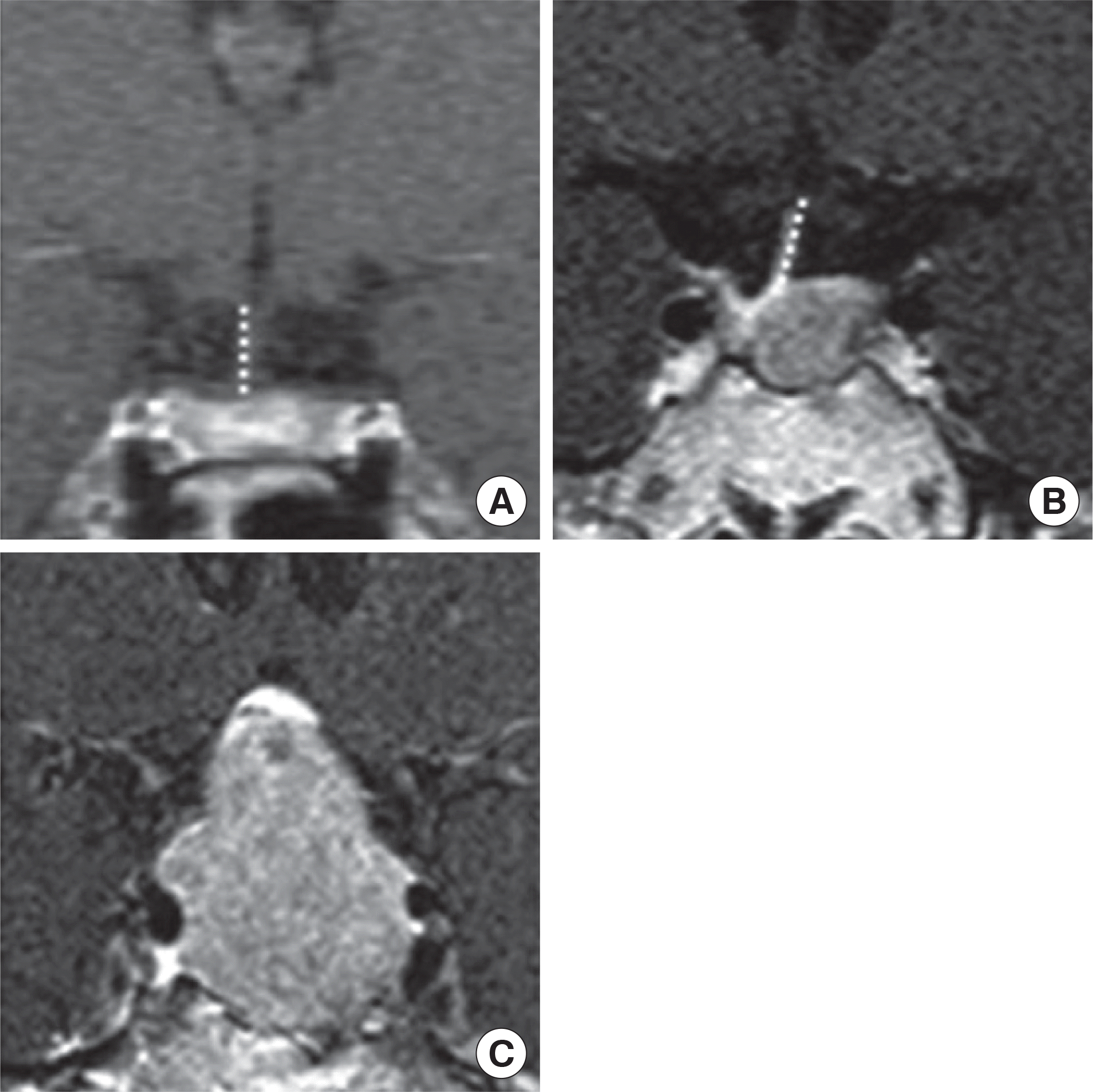 | Fig. 2.(A) An example case of normal stalk morphology. (B) An example case of stalk deviation; The stalk attachment of the pituitary gland deviated from the midline to the right side (dotted line). (C) An example case of stalk compression; Normal stalk morphology are not seen. |
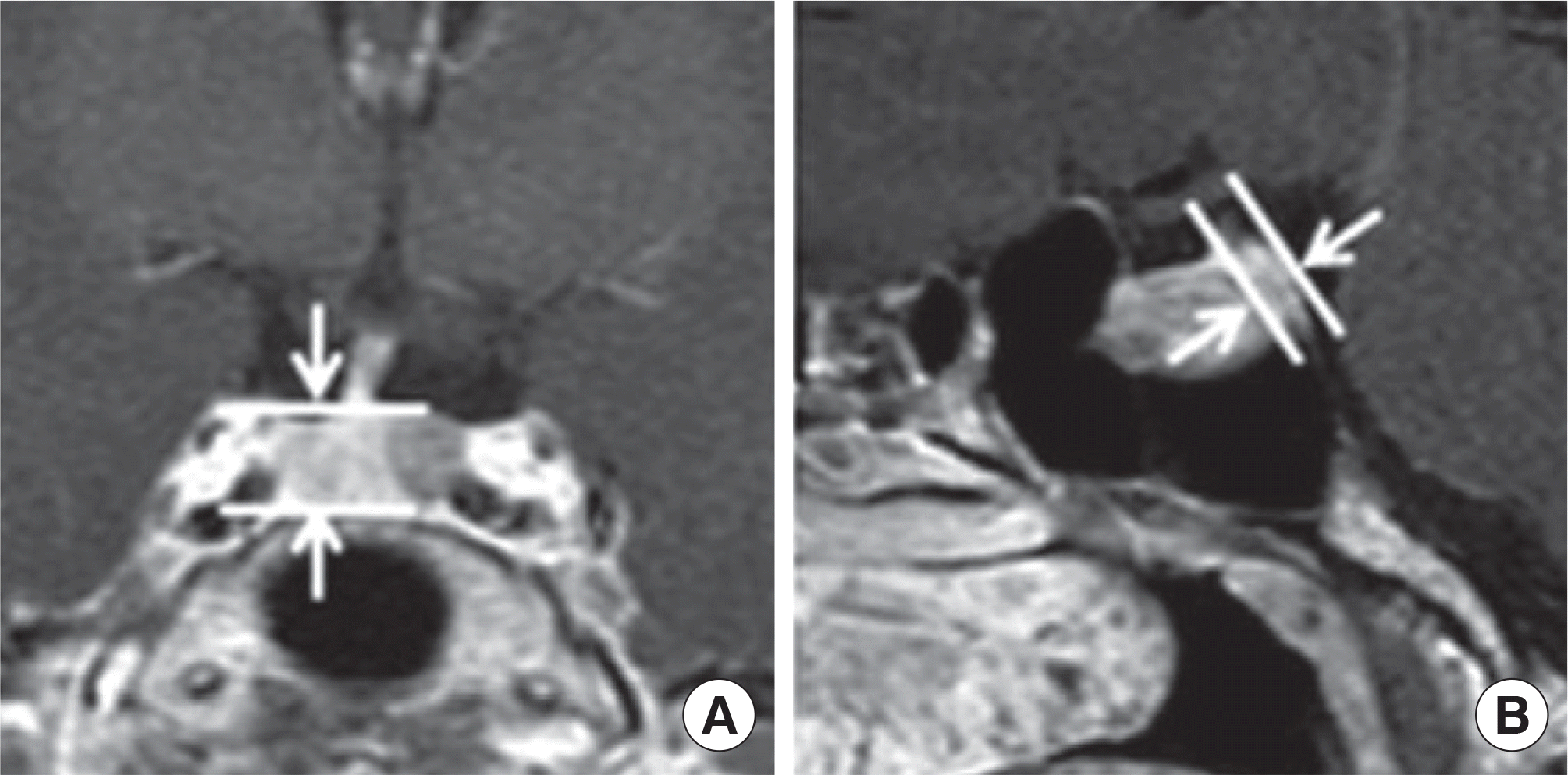 | Fig. 3.Measurement sites of the pituitary gland. (A) Maximal height were measured in coronal plane. (B) Maximal height were measured in sagittal plane. |
Table 1.
Interpretation of combined pituitary function test
Nadir glucose should be less than 40 mg/dL in nondiabetics or less than 50% of basal level in diabetics for appropriate stress induction. GH, growth hormone; ACTH, adrenocorticotropic hormone; i.v., intravenously; PRL, prolactin; TSH, thyroid-stimulating hormone; TRH, thyrotropin-releasing hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; LHRH, luteinizing hormone-releasing hormone; RI, regular insulin.
Table 2.
Baseline characteristics of 387 patients
Table 3.
Pituitary insufficiency in different age groups
| Age | Number of patients | Number of deficient hormone |
|---|---|---|
| < 20 | 4 | 1.75 ± 2.06 |
| 20 – 29 | 44 | 1.93 ± 1.60 |
| 30 – 39 | 66 | 2.33 ± 1.53 |
| 40 – 49 | 101 | 2.29 ± 1.46 |
| 50 – 59 | 107 | 2.69 ± 1.53 |
| 60 – 69 | 52 | 2.73 ± 1.49 |
| ≥ 70 | 13 | 3.85 ± 1.57* |
Table 4.
Pituitary Insufficiency depending on Tumor Size and Hardy's Classification
| Number of patients | Number of deficient hormone | ||
|---|---|---|---|
| Tumor size | < 10 mm | 9 | 0.44 ± 0.52 |
| 10 – 20 mm | 72 | 1.35 ± 1.42 | |
| 20 – 30 mm | 154 | 2.51 ± 1.53** | |
| 30 – 40 mm | 101 | 3.03 ± 1.29** | |
| 40 – 50 mm | 42 | 3.12 ± 1.23** | |
| ≥ 50 mm | 9 | 3.56 ± 1.33** | |
| Hardy's classification | I II IIIA | 21 58 101 | 1.19 ± 1.60 1.74 ± 1.58 2.88 ± 1.43** |
| IIIB | 153 | 2.65 ± 1.51** | |
| IV | 54 | 2.48 ± 1.35* |
Table 5.
Pituitary insufficiency depending on pituitary gland thickness an stalk morphology
| Number of patients | Number of deficient hormone | ||
|---|---|---|---|
| Thickness of | ≥ 5 mm | 29 | 1.21 ± 1.55 |
| pituitary gland | < 5 mm | 120 | 2.11 ± 1.57* |
| Not seen | 238 | 2.81 ± 1.45* | |
| Presence of stalk | Normal stalk | 40 | 1.18 ± 1.41 |
| compression | Stalk deviation | 146 | 2.29 ± 1.59* |
| Stalk compression | 201 | 2.87 ± 1.38* |
Table 6.
Multiple linear regression for variables independently associated with impaired pituitary function




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download