1. Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998; 21(Suppl 2):B161–B167. PMID:
9704245.
2. Kang HJ, Kwak HM, Kim YS, Park JS, Yoon G, Choi SJ, Oh SY, Kim JH, Roh CR. Obstetric and neonatal outcomes after treatment of gestational diabetes mellitus class A1 and class A2. Korean J Obstet Gynecol. 2010; 53:681–686.

3. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979; 28:1039–1057. PMID:
510803.
4. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005; 352:2477–2486. PMID:
15951574.
5. Kim MJ, Lee SK, Lee JA, Lee PR, Park HS. Risk factors for gestational diabetes mellitus in Korean women. Korean J Obes. 2013; 22:85–93.

6. Choi HM. Perinatal outcomes associated with prepregnancy body mass index and weight gain during pregnancy. Korean J Obstet Gynecol. 2010; 53:981–987.

7. Bray GA, Jablonski KA, Fujimoto WY, Barrett-Connor E, Haffner S, Hanson RL, Hill JO, Hubbard V, Kriska A, Stamm E, Pi-Sunyer FX. Diabetes Prevention Program Research Group. Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. Am J Clin Nutr. 2008; 87:1212–1218. PMID:
18469241.

8. Sadro CT, Dubinsky TJ. CT in pregnancy: risks and benefits. Appl Radiol. 2013; 42:6–16.
9. Bartha JL, Marin-Segura P, Gonzalez-Gonzalez NL, Wagner F, Aguilar-Diosdado M, Hervias-Vivancos B. Ultrasound evaluation of visceral fat and metabolic risk factors during early pregnancy. Obesity (Silver Spring). 2007; 15:2233–2239. PMID:
17890491.

10. Landy HJ, Gomez-Marin O, O'Sullivan MJ. Diagnosing gestational diabetes mellitus: use of a glucose screen without administering the glucose tolerance test. Obstet Gynecol. 1996; 87:395–400. PMID:
8598962.

11. World Health Organization. Regional Office for the Western Pacific. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia;2000.
12. Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol. 2007; 50:972–979. PMID:
17982340.

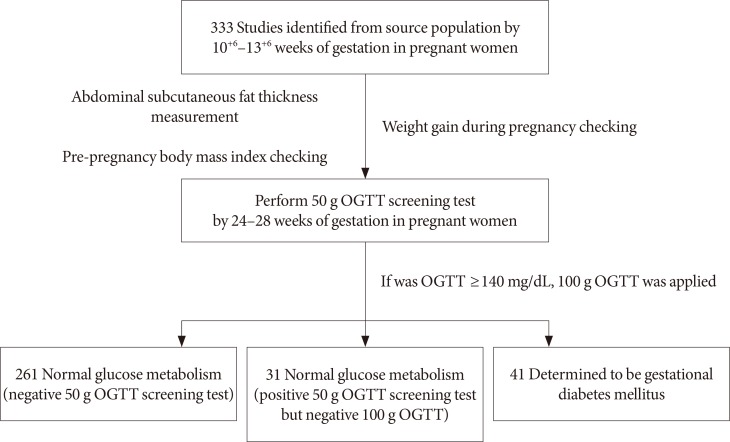
13. Park MI, Kim JJ, Lee JH, Kim SR, Ahn YH. The significance of 50 gm-oral glucose tolerance test in the screening of gestational diabetes mellitus. Korean J Obstet Gynecol. 1998; 41:2126–2130.
14. Makgoba M, Savvidou MD, Steer PJ. An analysis of the interrelationship between maternal age, body mass index and racial origin in the development of gestational diabetes mellitus. BJOG. 2012; 119:276–282. PMID:
22044452.

15. Philipsen A, Carstensen B, Sandbaek A, Almdal TP, Johansen NB, Jorgensen ME, Witte DR. Reproducibility of ultrasonography for assessing abdominal fat distribution in a population at high risk of diabetes. Nutr Diabetes. 2013; 3:e82. PMID:
23917154.

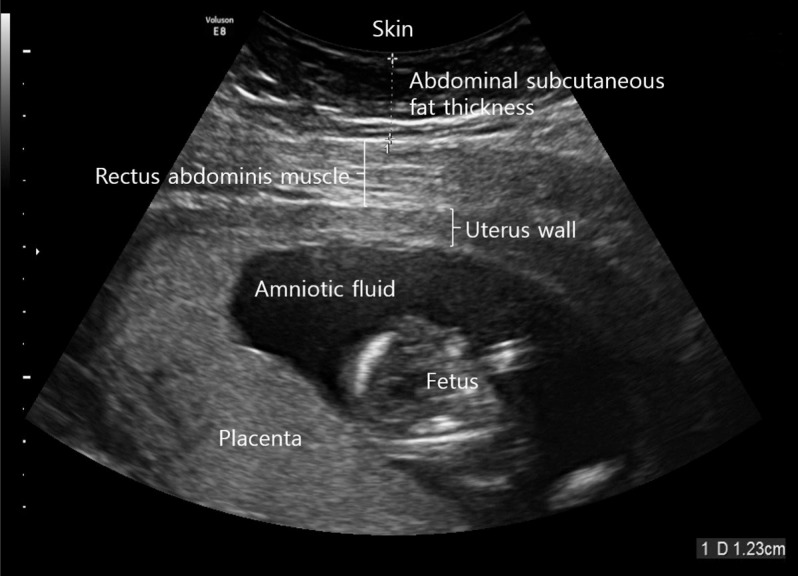
16. Kennedy NJ, Peek MJ, Quinton AE, Lanzarone V, Martin A, Benzie R, Nanan R. Maternal abdominal subcutaneous fat thickness as a predictor for adverse pregnancy outcome: a longitudinal cohort study. BJOG. 2016; 123:225–232. PMID:
26840907.

17. Gur EB, Ince O, Turan GA, Karadeniz M, Tatar S, Celik E, Yalcin M, Guclu S. Ultrasonographic visceral fat thickness in the first trimester can predict metabolic syndrome and gestational diabetes mellitus. Endocrine. 2014; 47:478–484. PMID:
24452873.

18. De Souza LR, Berger H, Retnakaran R, Maguire JL, Nathens AB, Connelly PW, Ray JG. First-trimester maternal abdominal adiposity predicts dysglycemia and gestational diabetes mellitus in midpregnancy. Diabetes Care. 2016; 39:61–64. PMID:
26525976.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download