Abstract
In order to improve the quality of life and to prevent chronic complications related to diabetes mellitus, intensive lifestyle modification and proper medication are needed from the early stage of diagnosis of type 2 diabetes mellitus (T2DM). When using the first medication for diabetic patients, the appropriate treatment should be selected considering the clinical characteristics of the patient, efficacy of the drug, side effects, and cost. In general, the use of metformin as the first treatment for oral hypoglycemic monotherapy is recommended because of its excellent blood glucose-lowering effect, relatively low side effects, long-term proven safety, low risk of hypoglycemia, and low weight gain. If metformin is difficult to use as a first-line treatment, other appropriate medications should be selected in view of the clinical situation. If the goal of achieving glycemic control is not achieved by monotherapy, a combination therapy with different mechanisms of action should be initiated promptly.
Many studies have shown that intensive control of blood glucose can significantly prevent diabetes-related chronic complications [12]. These results are the theoretical basis for explaining the need for active blood glucose management to improve the clinical course of diabetic patients. However, recent studies have reported that overly rigorous blood glucose control may lead to a negative clinical course in patients [345]. Individualized blood glucose control goals that take into account the diverse clinical situations of diabetic patients are required [67].
Lifestyle modification (LSM) is the first treatment for successful diabetes management. The effects of LSM on the clinical course of diabetes have been demonstrated in several studies [89]. However, due to the pathophysiological nature of type 2 diabetes mellitus (T2DM), where β-cell function is gradually diminishing, it is difficult to maintain adequate blood glucose control with LSM alone [10]. Therefore, in many patients, medication should be administered from the beginning of the treatment for proper blood glucose control.
This article was written to provide the rationale for the update of the position statement of the Korean Diabetes Association (KDA), and the contents of oral hypoglycemic agent monotherapy were described.
1. Active lifestyle modification and appropriate pharmacotherapy are needed from the initial diagnosis of diabetes [A].
2. An appropriate selection of pharmacotherapy should be made after considering the clinical characteristics of the patient and drug efficacy, side effects, mechanism of action, risk of hypoglycemia, effect on body weight, and patient preference and combined comorbidity [E].
1. Metformin is the preferred initial oral hypoglycemic agent [A].
2. If metformin is contraindicated or not well tolerated as the initial treatment, another class of hypoglycemic agent can be used depending on the clinical situation [E].
3. If monotherapy fails to achieve the glycemic target, combination therapy with a second agent with a different mechanism of action should be initiated [A].
In March 2017, the Clinical Practice Guideline (CPG) update was discussed at the Committee of Clinical Practice Guideline in the KDA. The committee decided to carry out an amendment in this revision that reflects the new diabetes medications. For this task, the committee formed a working group for revising the relevant content of the CPG. The guidelines were revised based on a systematic review of the newly published literature, along with the other national and international CPG contents. The details of this process are described in detail in other documents [11].
The task of the authors of the current article was evaluating the monotherapy of oral hypoglycemic agents for the working group. We have determined the key questions for revising the CPG according to the results of the discussion within the group. The first question is whether metformin is appropriate as a first-line choice for Korean patients with T2DM. The second question is how to choose the other first-line agent if metformin is not available. Finally, cardiovascular outcome with metformin or other monotherapy was determined to be the third key question.
For the purpose of revising the guidelines, various domestic and international guidelines have been referred to. We referred to the KDA guidelines and the Korea National Diabetes Program (KNDP) guidelines as domestic guidelines [1213] and referred to the guidelines of the American Diabetes Association (ADA), National Institute for Health and Care Excellence (NICE), International Diabetes Federation (IDF), Canadian Diabetes Association (CDA), and American Association of Clinical Endocrinologists and American College of Endocrinology (AACE) as foreign guidelines [1415161718]. References that meet our key questions were adopted. In addition, a systematic review was conducted to obtain the latest evidence. A master database for systematic review was built by professional librarians and delivered to group members. The evidence levels of the articles in the database were evaluated according to individual reviews of the group members. Thereafter, a list of articles was prepared by mutual review and agreement of group members. A final list was established by independent committee members separate from the working group [11].
The revised recommendations were circulated and evaluated by members of the committee other than the working group. Based on peer review, a draft CPG update was prepared. In July 2017, an initial draft was released at a public hearing. A final draft of the CPG update was prepared in accordance with the opinions gathered at the hearing. In August 2017, the final manuscript was approved by the Board of Directors, KDA.
For patients with T2DM who have not satisfactorily met therapeutic goals with LSM, a first-line oral hypoglycemic monotherapy should be administered. In monotherapy, approximately 0.5% to 1.5% of glycosylated hemoglobin (HbA1c) reduction is observed depending on the medication [19]. Although there are some differences depending on the class, the maximal effect of the drug is usually observed 4 to 6 months after treatment [20]. In general, the higher the patient's HbA1c, the greater the extent of HbA1c reduction with medication [19]. Postprandial glucose control becomes more important for further improvement of HbA1c when blood glucose approaches the generally recommended level (less than 7.3% of HbA1c) [21]. Some studies have shown that postprandial glucose is an independent risk factor for cardiovascular disease and death regardless of fasting glucose [2223]. However, the evidence for whether postprandial improvement of blood glucose is effective in improving additional cardiovascular disease outcomes is not yet clear.
Metformin is recommended as the drug for initial treatment in most diabetes-related CPGs worldwide [12131415161718]. Metformin is recommended as the first choice for patients with T2DM because of its excellent blood glucose-lowering effect, relatively low adverse effects, long-term safety, low risk of hypoglycemia, and low weight gain. These recommendations are based on a cohort study in which metformin monotherapy in overweight T2DM patients was associated with more marked blood glucose-lowering effects and less weight gain and hypoglycemia compared to sulfonylurea or insulin monotherapy [24]. Potential cardiovascular disease prevention effect is also included in the reason for choosing metformin as the initial treatment [2425]. However, the preventive effect of metformin on cardiovascular disease has yet to be ascertained.
In several subsequent observational studies and meta-analyses, there was evidence that metformin could be the drug of choice for initial treatment of diabetes patients compared to sulfonylurea, thiazolidinedione, and dipeptidyl peptidase 4 (DPP4) inhibitor, from the aspects of HbA1c reduction, side effects, weight gain, hypoglycemia, economic feasibility, and cardiovascular disease prevention [26272829]. In a prospective, multicenter clinical trial conducted in Korea, the effect of metformin monotherapy on HbA1c was similar to that of sulfonylurea or thiazolidinedione monotherapy [30]. Based on the above evidence, we also recommend metformin as an initial first-line medication in this CPG.
Clinical situations such as hepatic failure, chronic kidney disease (caution in estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2, contraindication in eGFR <30 mL/min/1.73 m2), severe infection, dehydration, and heart failure are contraindications of metformin use and it should be used with caution [1417]. Recently, a study suggesting that metformin use may be associated with vitamin B12 deficiency and anemia was published [31]. Vitamin B12 measurements may be considered for metformin users with peripheral neuropathy or anemia.
For patients who are contraindicated for metformin or who experience difficulties with metformin use, monotherapy of other hypoglycemic agents is considered as an initial treatment. Recently, as new drugs have been launched, various oral hypoglycemic agents have become available in clinical practice (Table 1) [11]. These drugs differ not only in their mechanism of action, but also in terms of cardiovascular disease prevention, side effects, contraindications, and price.
The DPP4 inhibitors have been widely used as a substitute for patients who have difficulty in using metformin monotherapy; these inhibitors are used because of their low incidence of side effects such as hypoglycemia. Recently, a meta-analysis has been reported by Korean researchers that suggests the effect of DPP4 inhibitors on Asians may be superior to other ethnicities [32]. The effects of the DPP4 inhibitors on cardiovascular disease have been reported to be neutral according to multicenter, prospective, randomized controlled trials performed recently performed trials [333435]. Although some DPP4 inhibitor have been reported to increase the risk of heart failure, systematic reviews have shown that the risk is not significant, and there is a slight difference in the risk of heart failure resulting from the use of DPP4 inhibitors [3637].
The use of sodium-glucose cotransporter 2 (SGLT2) inhibitors has recently led to a significant reduction in the risk of cardiovascular disease and mortality in patients with diabetes in multicenter, prospective, randomized controlled trials, and the frequency of use of these inhibitors is increasing in clinical settings [38394041]. However, due to possible side effects such as urogenital infection, dehydration, and hypotension, caution should be paid to its administration to some individuals such as the elderly and patients with chronic kidney disease [3840]. A recent multi-center, prospective, randomized controlled clinical trial has reported an increased risk of osteoporotic fractures and limb amputation associated with the use of this medication [41]. Further research on the long-term safety of this drug is needed.
A wide variety of previously used drugs such as sulfonylurea, meglitinide, thiazolidinedione, and α-glucosidase inhibitor can also be used as an effective substitute for metformin after securing various evidence on efficacy and safety when using this monotherapy [29424344]. There is some evidence that thiazolidinedione may reduce the cardiovascular disease risk in patients with T2DM who have a high risk of macrovascular events [4546]. However, attention should be paid to increased edema, anemia, bone fracture, and heart failure risk in patients [46]. α-Glucosidase inhibitor is an effective agent for postprandial glucose control [474849]; however, side effects such as gastrointestinal trouble are frequent, and there is a lack of evidence for cardiovascular outcome [4849]. Sulfonylurea and meglitinide have an excellent blood glucose-lowering effect. However, the cardiovascular benefit is not clear, and there is a risk of hypoglycemia [3050]. Recent studies in Korea have shown that hypoglycemia is closely related to adverse outcomes of patients [51525354]. Care should be taken with the use of drugs that are highly likely to cause hypoglycemia.
Diabetes treatment should be individualized according to the patient's needs and preferences, and drugs should be selected taking into account the specific advantages and disadvantages of each drug [7]. For a reasonable choice of medication, various clinical conditions should be considered including age, HbA1c, fasting and postprandial glucose, obesity or metabolic syndrome, insulin secretory capacity, risk of hypoglycemia, liver, cardiac or renal dysfunction, and patient preference.
Recently, new drugs have been introduced, and various clinical trials related to these drugs have been introduced. Different opinions on the selection of the initial treatment for patients with T2DM have been raised. We have yet to come to a complete conclusion as to which oral hypoglycemic agent should be the first choice for a particular patient, and which medication should be added next. In addition, we have not yet reached a consensus that it is reasonable to choose a particular medication for each of the various clinical situations. However, it is clinically more important to know what drug should control blood glucose, than what goal should blood glucose be controlled [17]. Even if blood glucose and HbA1c levels do not reach the target, the prognosis of the patient can be significantly improved depending on the degree of improvement of these levels [1].
Based on the literature review so far, metformin can be recommended as the first drug in Korean patients with T2DM. Metformin can be recommended from the variety of evidence accumulated to date. If metformin is difficult to use as an initial treatment, appropriate alternatives should be chosen considering the patient's individual circumstances. In addition to conventional drugs with which physicians have long-term experience such as sulfonylurea, thiazolidinedione, and α-glucosidase inhibitors, newer drugs such as the DPP4 inhibitors and the SGLT2 inhibitors also are indicated for monotherapy. If the goal of achieving glycemic control is not achieved by monotherapy, then combination therapy with different mechanisms of action should be initiated promptly.
ACKNOWLEDGMENTS
Financial support for the development of these guidelines was provided by the Korean Diabetes Association (KDA) operating budget; there was no support or involvement from industry sources.
This position statement on antihyperglycemic agent therapy was written by the KDA Committee of Clinical Practice Guidelines. We gratefully acknowledge the following experts who provided a critical review and discussion of this update: Tae-Nyun Kim, Inje University College of Medicine, Busan; Yong-ho Lee, Severance Hospital, Yonsei University College of Medicine, Seoul; Jin-Hwa Kim, Chosun University Hospital, Chosun University College of Medicine, Gwangju; Eun-Gyoung Hong, Hallym University Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong; Jaetaek Kim, Chung-Ang University College of Medicine, Seoul; Won-Young Lee, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul; Bokrye Song, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul; Ji Young Kim, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul; Dong Hee Yang, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang; Taeyoung Yang, Taeyoung 21 Hospital, Gwangju; and Hyeongjin Kim, Kim HJ Medical Clinic, Paju, Korea.
Notes
References
1. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998; 352:837–853. PMID: 9742976.
2. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008; 359:1577–1589. PMID: 18784090.

3. Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559. PMID: 18539917.
4. ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560–2572. PMID: 18539916.
5. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009; 360:129–139. PMID: 19092145.

6. Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011; 154:554–559. PMID: 21502652.

7. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015; 38:140–149. PMID: 25538310.

8. Look AHEAD Research Group. Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013; 369:145–154. PMID: 23796131.
9. Pillay J, Armstrong MJ, Butalia S, Donovan LE, Sigal RJ, Vandermeer B, Chordiya P, Dhakal S, Hartling L, Nuspl M, Featherstone R, Dryden DM. Behavioral programs for type 2 diabetes mellitus: a systematic review and network meta-analysis. Ann Intern Med. 2015; 163:848–860. PMID: 26414227.
10. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999; 281:2005–2012. PMID: 10359389.
11. Ko SH, Hur KY, Rhee SY, Kim NH, Moon MK, Park SI, Lee BW, Kim HJ, Choi KM, Kim JH. Committee of Clinical Practice Guideline of Korean Diabetes Association. Antihyperglycemic agent therapy for adult patients with type 2 diabetes mellitus 2017: a position statement of the Korean Diabetes Association. Diabetes Metab J. 2017; 41:337–348.

12. The Korea National Diabetes Program (KNDP) Investigators. Clinical practice guideline for the prevention and management of diabetes in Korea. Seoul: Medbook;2014.
13. Korean Diabetes Association. 2015 Treatment guidelines for diabetes. Seoul: Korean Diabetes Association;2015.
14. American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2017; 40(Suppl 1):S64–S74. PMID: 27979895.
15. McGuire H, Longson D, Adler A, Farmer A, Lewin I. Guideline Development Group. Management of type 2 diabetes in adults: summary of updated NICE guidance. BMJ. 2016; 353:i1575. PMID: 27052837.

16. International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014; 104:1–52. PMID: 24508150.
17. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Harper W, Clement M, Goldenberg R, Hanna A, Main A, Retnakaran R, Sherifali D, Woo V, Yale JF. Pharmacologic management of type 2 diabetes. Can J Diabetes. 2013; 37(Suppl 1):S61–S68. PMID: 24070965.

18. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA, Einhorn D, Fonseca VA, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez GE. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm: 2017 executive summary. Endocr Pract. 2017; 23:207–238. PMID: 28095040.
19. Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE. Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: a meta-regression analysis. Diabetes Care. 2006; 29:2137–2139. PMID: 16936168.
20. Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes Care. 2010; 33:1859–1864. PMID: 20484130.
21. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003; 26:881–885. PMID: 12610053.
22. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in type II diabetes: the epidemiological evidence. Diabetologia. 2001; 44:2107–2114. PMID: 11793012.

23. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes epidemiology: collaborative analysis of diagnostic criteria in Europe. Lancet. 1999; 354:617–621. PMID: 10466661.
24. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998; 352:854–865. PMID: 9742977.
25. Goldberg RB, Aroda VR, Bluemke DA, Barrett-Connor E, Budoff M, Crandall JP, Dabelea D, Horton ES, Mather KJ, Orchard TJ, Schade D, Watson K, Temprosa M. Diabetes Prevention Program Research Group. Effect of long-term metformin and lifestyle in the diabetes prevention program and its outcome study on coronary artery calcium. Circulation. 2017; 136:52–64. PMID: 28476766.

26. Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, Marinopoulos SS, Puhan MA, Ranasinghe P, Block L, Nicholson WK, Hutfless S, Bass EB, Bolen S. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011; 154:602–613. PMID: 21403054.

27. Chan JC, Deerochanawong C, Shera AS, Yoon KH, Adam JM, Ta VB, Chan SP, Fernando RE, Horn LC, Nguyen TK, Litonjua AD, Soegondo S, Zimmet P. Role of metformin in the initiation of pharmacotherapy for type 2 diabetes: an Asian-Pacific perspective. Diabetes Res Clin Pract. 2007; 75:255–266. PMID: 16876285.

28. Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, Chu Y, Iyoha E, Segal JB, Bolen S. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016; 164:740–751. PMID: 27088241.
29. Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, Maggo J, Gray V, De Berardis G, Ruospo M, Natale P, Saglimbene V, Badve SV, Cho Y, Nadeau-Fredette AC, Burke M, Faruque L, Lloyd A, Ahmad N, Liu Y, Tiv S, Wiebe N, Strippoli GF. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. JAMA. 2016; 316:313–324. PMID: 27434443.
30. Yoon KH, Shin JA, Kwon HS, Lee SH, Min KW, Ahn YB, Yoo SJ, Ahn KJ, Park SW, Lee KW, Sung YA, Park TS, Kim MS, Kim YK, Nam MS, Kim HS, Park IeB, Park JS, Woo JT, Son HY. Comparison of the efficacy of glimepiride, metformin, and rosiglitazone monotherapy in Korean drug-naive type 2 diabetic patients: the practical evidence of antidiabetic monotherapy study. Diabetes Metab J. 2011; 35:26–33. PMID: 21537410.
31. Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, Orchard TJ, Bray GA, Schade DS, Temprosa MG, White NH, Crandall JP. Diabetes Prevention Program Research Group. Long-term metformin use and vitamin B12 deficiency in the diabetes prevention program outcomes study. J Clin Endocrinol Metab. 2016; 101:1754–1761. PMID: 26900641.

32. Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013; 56:696–708. PMID: 23344728.

33. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013; 369:1317–1326. PMID: 23992601.

34. White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F. EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013; 369:1327–1335. PMID: 23992602.

35. Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR. TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015; 373:232–242. PMID: 26052984.

36. Abbas AS, Dehbi HM, Ray KK. Cardiovascular and non-cardiovascular safety of dipeptidyl peptidase-4 inhibition: a meta-analysis of randomized controlled cardiovascular outcome trials. Diabetes Obes Metab. 2016; 18:295–299. PMID: 26510994.

37. Kongwatcharapong J, Dilokthornsakul P, Nathisuwan S, Phrommintikul A, Chaiyakunapruk N. Effect of dipeptidyl peptidase-4 inhibitors on heart failure: a meta-analysis of randomized clinical trials. Int J Cardiol. 2016; 211:88–95. PMID: 26991555.

38. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–2128. PMID: 26378978.

39. Monami M, Dicembrini I, Mannucci E. Effects of SGLT2 inhibitors on mortality and cardiovascular events: a comprehensive meta-analysis of randomized controlled trials. Acta Diabetol. 2017; 54:19–36. PMID: 27488726.

40. Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ. EMPA-REG OUTCOME(R) Investigators. Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: results from EMPA-REG OUTCOME((R)). Circ J. 2017; 81:227–234. PMID: 28025462.
41. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377:644–657. PMID: 28605608.

42. Varvaki Rados D, Catani Pinto L, Reck Remonti L, Bauermann Leitao C, Gross JL. The association between sulfonylurea use and all-cause and cardiovascular mortality: a meta-analysis with trial sequential analysis of randomized clinical trials. PLoS Med. 2016; 13:e1001992. PMID: 27071029.

43. Kim SY, Kim HJ, Han KA, Baek SH, Son HS, Cha BS, Min KW. Efficacy and safety of mitiglinide in Korean type 2 diabetic patients: prospective randomised multicenter comparative phase III study. J Korean Diabetes Assoc. 2007; 31:163–174.

44. Joshi SR, Standl E, Tong N, Shah P, Kalra S, Rathod R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expert Opin Pharmacother. 2015; 16:1959–1981. PMID: 26255950.

45. Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomized controlled trial. Lancet. 2005; 366:1279–1289. PMID: 16214598.
46. Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007; 298:1180–1188. PMID: 17848652.
47. Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, Ross SA, Ryan EA, Tan MH, Wolever TM. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical trial. Ann Intern Med. 1994; 121:928–935. PMID: 7734015.

48. Hoffmann J, Spengler M. Efficacy of 24-week monotherapy with acarbose, glibenclamide, or placebo in NIDDM patients. The Essen Study. Diabetes Care. 1994; 17:561–566. PMID: 8082525.
49. Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (U.K. Prospective Diabetes Study 44). Diabetes Care. 1999; 22:960–964. PMID: 10372249.

50. Black C, Donnelly P, McIntyre L, Royle PL, Shepherd JP, Thomas S. Meglitinide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007; (2):CD004654. PMID: 17443551.

51. Chin SO, Rhee SY, Chon S, Baik SH, Park Y, Nam MS, Lee KW, Chun KH, Woo JT, Kim YS. Hypoglycemia is associated with dementia in elderly patients with type 2 diabetes mellitus: an analysis based on the Korea National Diabetes Program Cohort. Diabetes Res Clin Pract. 2016; 122:54–61. PMID: 27810686.

52. Rhee SY, Hong SM, Chon S, Ahn KJ, Kim SH, Baik SH, Park YS, Nam MS, Lee KW, Woo JT, Kim YS. Hypoglycemia and medical expenses in patients with type 2 diabetes mellitus: an analysis based on the Korea National Diabetes Program Cohort. PLoS One. 2016; 11:e0148630. PMID: 26890789.

53. Cha SA, Yun JS, Lim TS, Hwang S, Yim EJ, Song KH, Yoo KD, Park YM, Ahn YB, Ko SH. Severe hypoglycemia and cardiovascular or all-cause mortality in patients with type 2 diabetes. Diabetes Metab J. 2016; 40:202–210. PMID: 27098504.

54. Yun JS, Ko SH. Risk factors and adverse outcomes of severe hypoglycemia in type 2 diabetes mellitus. Diabetes Metab J. 2016; 40:423–432. PMID: 27766794.

Table 1
Oral antihyperglycemic agents for patients with type 2 diabetes mellitus used in Korea
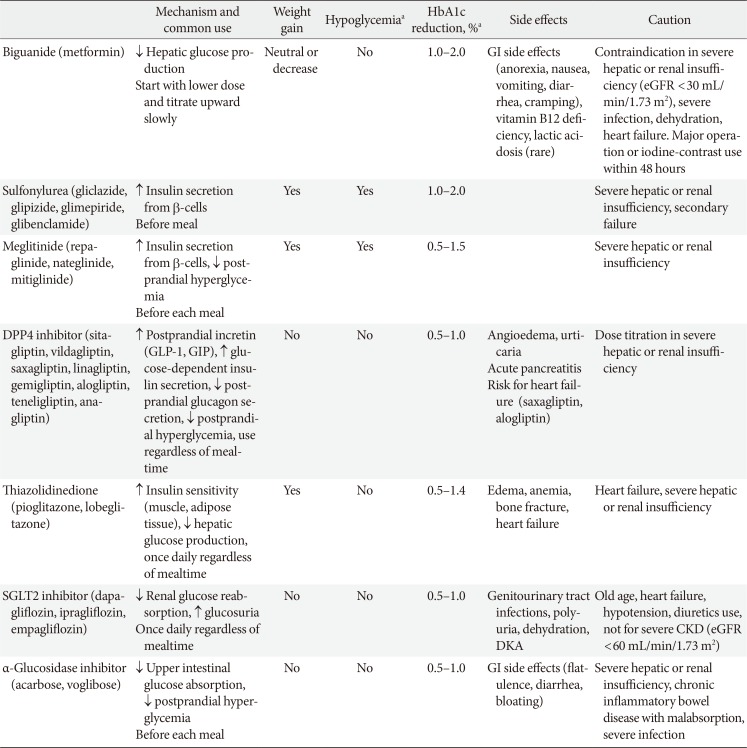
Adapted Ko et al. [11].
HbA1c, glycosylated hemoglobin; GI, gastrointestinal; eGFR, estimated glomerular filtration rate; DPP4, dipeptidyl peptidase 4; GLP-1, glucagon-like peptide 1; GIP, gastric inhibitory polypeptide; CKD, chronic kidney disease; SGLT2, sodium-glucose cotransporter 2; DKA, diabetic ketoacidosis.
aMonotherapy.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download