Abstract
Background
The aim of this study was to evaluate the safety and effectiveness of insulin glargine in a large population from a variety of clinical care in Iranian people with type 2 diabetes mellitus (T2DM) and to measure the percentage of patients achieving glycosylated hemoglobin (HbA1c) <7% by the end of 24 weeks of treatment in routine clinical practice.
Methods
This study was a 24 week, observational study of patients with T2DM, for whom the physician had decided to initiate or to switch to insulin glargine. The safety and efficacy of glargine were assessed at baseline and at week 24.
Results
Seven hundred and twenty-five people with T2DM (63% female) including both insulin naïve and prior insulin users were recruited in this study. The mean age of the participants was 54.2±11.2 years, and the mean HbA1c level was 8.88%±0.93% at baseline. By the end of the study, 27% of the entire participants reached to HbA1c target of less than 7% and 52% had HbA1c ≤7.5%. No serious adverse event was reported in this study. Furthermore, overall hypoglycemia did not increase in prior insulin users and the entire cohort. In addition, body weight did not change in participants while lipid profile improved significantly.
Diabetes is rising globally and its prevalence has doubled over nearly three decades [1]. In patients with type 2 diabetes mellitus (T2DM), prolonged hyperglycemia is associated with long-term microvascular and macrovascular complications [234]. It has been demonstrated that maintaining tight glycemic control decreases the risks of long-term complications, as well as mortality and morbidity [5678]. However, tight glycemic control is associated with an increased risk of hypoglycemia [89] that often results in poor compliance with treatment and subsequent failure to achieve targeted glycosylated hemoglobin (HbA1c) levels [10]. Despite the availability of a number of treatment strategies such as proper diets, lifestyle modification, oral glucose lowering drugs, and supplement adjuvants, the majority of patients with T2DM are not currently meeting the recommended treatment goals [51112]. If lifestyle intervention and intensive treatment with oral lowering glucose drugs (OGLDs) fail to achieve the targeted HbA1c levels, basal insulin therapy is recommended [13]. Newer insulin therapies such as different types of insulin analogues trying to simulate the physiologic basal-prandial insulin have changed clinical diabetes care [1415]. Insulin glargine (LANTUS; Sanofi-Aventis, Paris, France) is a basal insulin analogue with no pronounced peak, consistent activity profile [16], and lower risk of nocturnal hypoglycemia compared with neutral protamine Hagedorn (NPH) insulin in patients with T2DM [2]. It has been shown that insulin glargine can be used to treat to glycemic targets <7.0% in insulin naïve patients [1718]. The aim of this study was, therefore, to broaden the knowledge of the clinical safety and effectiveness of insulin glargine in a large population from a variety of clinical care in Iranian people with T2DM and to measure the percentage of patients achieving HbA1c <7% by the end of 24 weeks of treatment.
This was a 24-week nation-wide, prospective, multicenter, observational study of people with T2DM who had been initiated or switched to insulin glargine in order to evaluate its clinical safety and effectiveness in routine clinical practice. The study was carried out in 70 centers in Iran. Participants were recruited between March 2011 and March 2012. The physician took the independent decision to initiate (or switch) insulin glargine as basal insulin therapy as part of the routine clinical care. Changes to OGLDs at the time of starting insulin glargine, or thereafter, were entirely at the discretion of the advising physician. Verbal and written instructions were provided to the patient on how to perform self-monitoring of blood glucose levels, how to self-titrate insulin glargine, and how to fill in their individual diary.
According to the American Diabetes Association/European Association for the Study of Diabetes Consensus Algorithm, and as part of usual clinical care, the patient had an assessment of HbA1c every 3 months until the target of <7% was reached. Thus, each patient included in this study had an HbA1c determination at baseline (at most 2 months before inclusion), 12 weeks after insulin initiation and at the end of the study (week 24). HbA1c determination was performed in the same laboratory for a given patient. Data were collected from the physicians' clinical notes and participants' recall and self-monitoring diary/meter at each visit, as available. This information was transferred to a standard case report forms.
Seven hundred and twenty-five people with T2DM, >18 years of age, for whom the physician had decided to initiate or to switch to insulin glargine were included in the study. Subjects had HbA1c >7.0% but <10%. Eligible participants had the diagnosis of T2DM for at least 1 year.
Subjects were excluded for any of the following criteria: pregnant or lactating women, patients already treated with insulin other than insulin NPH, patients treated with OGLDs other than Metformin, Sulfonylurea or Pioglitazone, patients not willing, or not able, to perform self-monitoring blood glucose, patients not willing, or not able, to self-titrate insulin glargine under physician's guidance, patients with any serious underlying illness, including hepatic failure (aspartate aminotransferase test >3 upper limit of normal range), renal failure (creatinine >1.5 mg/dL in males and >1.4 mg/dL in females), psychiatric patients, patients with history of drug or alcohol abuse and patients on chronic treatment with systemic corticosteroids, hospitalized patients, patient who were involved in other clinical trials, patients who were not suitable for close follow-up, and known hypersensitivity to insulin glargine. Ethics Committee Approval was obtained from Iran University of Medical Sciences. All participants signed informed consent from. In addition, they were free to withdraw at any time.
The primary objective of this study was to measure the percentage of patients achieving HbA1c <7% by the end of the study (week 24). The efficacy was also assessed as follows: the percentage of patients achieving HbA1c <7%, the changes in HbA1c at week 24 compared to baseline HbA1c, the changes of basal insulin doses at week 24 (average dosage per patient and timing), and the changes in body weight and lipid profile from baseline.
Safety assessment were the changes in the number of hypoglycemic episodes (blood glucose levels of less than 60 mg/dL [3.3 mmol/L]) occurred in preceding 4 weeks prior to enrollment, and during the study, the changes in the number of nocturnal hypoglycemic events and the number of adverse drug reactions (ADRs) from baseline to final visit.
Major hypoglycemic events were defined as events with symptoms of hypoglycemia for which subjects required the assistance of another person, and were associated with a blood glucose level below 60 mg/dL (3.3 mmol/L) or prompt recovery after oral carbohydrate consumption, intravenous glucose, or glucagon administration. Minor hypoglycemia was any event, where symptoms consistent with hypoglycemia were experienced and either the subjects responded to ingestion of carbohydrate/snack or the episode was associated with a glycemia below 60 mg/dL (3.3 mmol/L).
IBM SPSS version 19 (IBM Co., Armonk, NY, USA) was used for the statistical analysis. The data were analyzed anonymously. Based on the assumption that 30% of subjects would have an HbA1c <7% at week 24, it was planned to include a total sample of 659 evaluable patients to measure this rate with a 95% confidence interval of ±3.5%. Assuming a 10% drop-out rate, the total number of subjects to be recruited in the study was at least 725.
Analyses were performed on all patients who finished the study according to prior insulin use. Continuous variables were summarized using number of patients, mean±standard deviation, and discrete variables were summarized using number of patients and percentage. Hypoglycemia were summarized as number of events per patient-year, All statistical tests were two-sided, and a P<0.05 was considered statistically significant. For hypoglycemia change from baseline, the percentage of people reporting at least one event was analyzed using McNemar's test. Change from baseline HbA1c, fasting plasma glucose (FPG), and blood lipids was analyzed using paired sample t-test. The percentage of patients having HbA1c <7.0% at 24 weeks was reported using descriptive statistics.
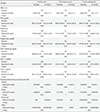
Seven hundred and twenty-three subjects were included in this study. Four hundred and fifty-three (63%) were female. The mean age was 54.2±11.2 years, and the mean duration of diabetes was 9.5±6.3 years. Table 1 shows baseline characteristics of insulin naïve, prior insulin users, and entire participants. Use of OGLDs prior to initiating insulin glargine is also demonstrated in Table 1. Regarding medication, 89.8% of patients were being treated with OGLDs alone, 6.9% were receiving OGLD+insulin therapy, and 3.3% were on insulin only.
With regards to prior insulin treatment, insulin-treated people were the same age as insulin naïve patients and they had similar BMI (27.99 kg/m2 vs. 27.84 kg/m2). Six hundred and thirty-nine patients completed the study. Baseline HbA1c was lower (8.7% vs. 8.9%) in prior insulin users, while diabetes duration was longer (11.2 years vs. 9.4 years).
Blood glucose improved significantly by week 24. The HbA1c dropped from 8.88%±0.93% at baseline to 7.62%±1.17% in the whole participants. This improvement was also observed in both the insulin naïve and prior insulin users (Table 2). By the end of the study 27% of the entire participants reached to HbA1c target of less than 7% and 52% had HbA1c ≤7.5%.
Total daily insulin dose had been titrated up to 20.32±10.31 U/day at week 24 in people who were insulin naïve. In prior insulin users, the starting insulin dose was 22.98±12.02 U/day titrating up to 30.24±13.65 U/day at week 24.
After initiating insulin in insulin naïve people, metformin was continued in around 86% of people. While 41.4% of people starting insulin glargine continued using sulfonylureas and 64% discontinued thiazolidinedione.
In prior insulin users, metformin was discontinued in about 6% of people, 68.7% continued sulfonylurea while thiazolidinedione was continued in all prior insulin users. The changes in mean body weight were neither statistically nor clinically significant over 24 weeks for the total participants, the insulin-naïve population, and prior insulin users (Table 2).
Total cholesterol level reduced in the whole participants from a mean of 189.96 to 174.98 mg/dL after 24 weeks (P<0.001) (Table 2). A significant reduction was seen in low density lipoprotein cholesterol level in the entire cohort and insulin-naïve people.
Triglyceride levels also dropped from a mean of 187.94 to 160.21 mg/dL in total participants (P<0.001). Results were similar for the insulin-naïve people and prior insulin users. Furthermore, there was a small increase in high density lipo-protein cholesterol levels during the study in the entire population and insulin-naïve people.
The reported rate of overall hypoglycemic episodes did not differ significantly between baseline and week 24 in the entire cohort and prior insulin users while it was statistically different in the insulin naïve people (Table 2). In the whole participants reported rates of overall hypoglycemia increased from 0.08 to 0.12 events/person-year, with no statistical difference in the proportion of people having an event. In the prior insulin users, the reported rate of hypoglycemia decreased from 0.16 to 0.13 events/person-year by the end of the study, but it was not statistically significant (P=0.99). In Insulin naïve patients, this number increased from 0.06 to 0.09 events/person-year (P=0.03). The rates of minor, nocturnal, and major hypoglycemic events remained clinically and statistically unchanged in the entire participants (Table 2).
The Iran Affect Study was a 24-week, nation-wide, observational study in patients with T2DM, conducted to measure the percentage of patients achieving HbA1c <7% at the end of 6 months period using insulin glargine in routine clinical practice in Iran. The safety and efficacy of insulin glargine were also assessed at baseline and at week 24. The results achieved in this study showed that initiating or switching insulin glargine was safe and effective with marked improvements in metabolic control (as measured by glucose and lipid profile); these results are consistent with previous studies [192021]. These findings reflect the beneficial effects of receiving insulin glargine as good glycemic control is associated with a reduced risk of chronic micro and macrovascular complications [818] as well as decreasing overall mortality and morbidity [1722]. In addition, changes in body weight were neither statistically nor clinically significant. This result is in concordance with the findings in randomized clinical trials [251423]. The neutral effect of glargine on body weight in this study may be due to increased self-care behaviors, improvement in lifestyle and nutritional status of the participants [24] suggesting that insulin glargine may be more beneficial in treating obese patients with T2DM [25].
Treatment with insulin glargine leads to a reduction of HbA1c to 7.62% after 24 weeks of treatment. In addition, FPG decreased significantly from baseline. Notably, the large reduction in the HbA1c and FPG levels following 24 weeks of use of insulin glargine was associated with a low incidence of reported serious ADRs and hypoglycemia. We found no increase in the reported rate of minor, major, and nocturnal hypoglycemic episodes due to the fact that insulin glargine as a basal insulin analogue has consistent daily activity profile with no pronounced peak and low risk of nocturnal hypoglycemia [26]. It also typically shows a gentle rise and fall in glucose-lowering action over time with reduced variability [27] that mimics normal physiologic insulin concentrations [28].
We also observed improvement in lipid profile in people with T2DM. Although it might be due to better glucose control with insulin glargine, changing concomitant medications, dietary intake and lifestyle are some other factor that can affect the lipid profile which were not controlled in this study.
As this was an observational study, there were some other limitations; the circumstances under which participants came under the care of the investigators were not known due to the nature of the study and there were not control group and standard treatment protocol.
In routine clinical practice, treatment with insulin glargine was effective and safe in Iranian people with T2DM. This improvement in metabolic control was observed in insulin naïve as well as prior insulin users. As diabetes and dyslipidemia significantly increase the risk of cardiovascular disease, improving metabolic outcomes might be effective on reducing diabetes chronic complications and subsequent morbidity and mortality.
Figures and Tables
Table 1
Baseline characteristics for the entire cohort and by pre-study therapy

Table 2
Glucose control and body weight for the entire participants and by pre-study therapy at baseline and after 24 weeks of insulin glargine therapy
References
1. Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011; 378:31–40.
2. Eliaschewitz FG, Calvo C, Valbuena H, Ruiz M, Aschner P, Villena J, Ramirez LA, Jimenez J. HOE 901/4013 LA Study Group. Therapy in type 2 diabetes: insulin glargine vs. NPH insulin both in combination with glimepiride. Arch Med Res. 2006; 37:495–501.
3. Malek M, Khamseh ME, Aghili R, Emami Z, Najafi L, Baradaran HR. Medical management of diabetic retinopathy: an overview. Arch Iran Med. 2012; 15:635–640.
4. Khamseh ME, Kazemikho N, Aghili R, Forough B, Lajevardi M, Hashem Dabaghian F, Goushegir A, Malek M. Diabetic distal symmetric polyneuropathy: effect of low-intensity laser therapy. Lasers Med Sci. 2011; 26:831–835.
5. Duckworth W, Davis SN. Comparison of insulin glargine and NPH insulin in the treatment of type 2 diabetes: a review of clinical studies. J Diabetes Complications. 2007; 21:196–204.
6. Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004; 141:413–420.
7. Khaw KT, Wareham N, Luben R, Bingham S, Oakes S, Welch A, Day N. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of european prospective investigation of cancer and nutrition (EPIC-Norfolk). BMJ. 2001; 322:15–18.
8. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321:405–412.
9. Lawson ML, Gerstein HC, Tsui E, Zinman B. Effect of intensive therapy on early macrovascular disease in young individuals with type 1 diabetes. A systematic review and meta-analysis. Diabetes Care. 1999; 22:Suppl 2. B35–B39.
10. Fritsche A, Schweitzer MA, Haring HU. 4001 Study Group. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med. 2003; 138:952–959.
11. Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004; 291:335–342.
12. Farshchi A, Nikfar S, Abdollahi M. Concerns on the use of chromium in type 2 diabetes mellitus: needs to conduct major meta-analysis. Int J Pharmacol. 2012; 8:470–472.
13. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. American Diabetes Association. European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009; 32:193–203.
14. Yokoyama H, Tada J, Kamikawa F, Kanno S, Yokota Y, Kuramitsu M. Efficacy of conversion from bedtime NPH insulin to morning insulin glargine in type 2 diabetic patients on basal-prandial insulin therapy. Diabetes Res Clin Pract. 2006; 73:35–40.
15. DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003; 289:2254–2264.
16. Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000; 23:644–649.
17. Riddle MC, Rosenstock J, Gerich J. Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003; 26:3080–3086.
18. American Diabetes Association. Standards of medical care in diabetes: 2013. Diabetes Care. 2013; 36:Suppl 1. S11–S66.
19. Yki-Jarvinen H, Dressler A, Ziemen M. HOE 901/300s Study Group. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000; 23:1130–1136.
20. Yki-Jarvinen H, Kauppinen-Makelin R, Tiikkainen M, Vahatalo M, Virtamo H, Nikkila K, Tulokas T, Hulme S, Hardy K, McNulty S, Hanninen J, Levanen H, Lahdenpera S, Lehtonen R, Ryysy L. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006; 49:442–451.
21. Rosenstock J, Dailey G, Massi-Benedetti M, Fritsche A, Lin Z, Salzman A. Reduced hypoglycemia risk with insulin glargine: a meta-analysis comparing insulin glargine with human NPH insulin in type 2 diabetes. Diabetes Care. 2005; 28:950–955.
22. Wright A, Burden AC, Paisey RB, Cull CA, Holman RR. U.K. Prospective Diabetes Study Group. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002; 25:330–336.
23. Pan CY, Sinnassamy P, Chung KD, Kim KW. LEAD Study Investigators Group. Insulin glargine versus NPH insulin therapy in Asian type 2 diabetes patients. Diabetes Res Clin Pract. 2007; 76:111–118.
24. Home P, Naggar NE, Khamseh M, Gonzalez-Galvez G, Shen C, Chakkarwar P, Wenying Y. An observational non-interventional study of people with diabetes beginning or changed to insulin analogue therapy in non-Western countries: the A1chieve study. Diabetes Res Clin Pract. 2011; 94:352–363.
25. Massi Benedetti M, Humburg E, Dressler A, Ziemen M. A one-year, randomised, multicentre trial comparing insulin glargine with NPH insulin in combination with oral agents in patients with type 2 diabetes. Horm Metab Res. 2003; 35:189–196.
26. Lepore M, Pampanelli S, Fanelli C, Porcellati F, Bartocci L, Di Vincenzo A, Cordoni C, Costa E, Brunetti P, Bolli GB. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000; 49:2142–2148.
27. Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab. 2007; 9:648–659.
28. Owens DR, Coates PA, Luzio SD, Tinbergen JP, Kurzhals R. Pharmacokinetics of 125I-labeled insulin glargine (HOE 901) in healthy men: comparison with NPH insulin and the influence of different subcutaneous injection sites. Diabetes Care. 2000; 23:813–819.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download