1. Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012; 379:2279–2290. PMID:
22683128.
2. Forouhi NG, Luan J, Hennings S, Wareham NJ. Incidence of type 2 diabetes in England and its association with baseline impaired fasting glucose: the Ely study 1990-2000. Diabet Med. 2007; 24:200–207. PMID:
17257284.

3. Horseman ND. Prolactin and mammary gland development. J Mammary Gland Biol Neoplasia. 1999; 4:79–88. PMID:
10219908.
4. Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extra-pituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev. 1996; 17:639–669. PMID:
8969972.

5. Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000; 80:1523–1631. PMID:
11015620.
6. Ben-Jonathan N, Hugo ER, Brandebourg TD, LaPensee CR. Focus on prolactin as a metabolic hormone. Trends Endocrinol Metab. 2006; 17:110–116. PMID:
16517173.

7. Huang C, Snider F, Cross JC. Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology. 2009; 150:1618–1626. PMID:
19036882.
8. Labriola L, Montor WR, Krogh K, Lojudice FH, Genzini T, Goldberg AC, Eliaschewitz FG, Sogayar MC. Beneficial effects of prolactin and laminin on human pancreatic islet-cell cultures. Mol Cell Endocrinol. 2007; 263:120–133. PMID:
17081683.

9. Wang T, Lu J, Xu Y, Li M, Sun J, Zhang J, Xu B, Xu M, Chen Y, Bi Y, Wang W, Ning G. Circulating prolactin associates with diabetes and impaired glucose regulation: a population-based study. Diabetes Care. 2013; 36:1974–1980. PMID:
23340889.
10. Haring R, Volzke H, Vasan RS, Felix SB, Nauck M, Dorr M, Wallaschofski H. Sex-specific associations of serum prolactin concentrations with cardiac remodeling: longitudinal results from the Study of Health Pomerania (SHIP). Atherosclerosis. 2012; 221:570–576. PMID:
22293228.

11. Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010; 122:570–578. PMID:
20660804.
12. Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, Wong ND, Smith VE, Gottdiener J. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study). Am J Cardiol. 2001; 87:1051–1057. PMID:
11348601.

13. World Health Organization. International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. Geneva: World Health Organization;2006.
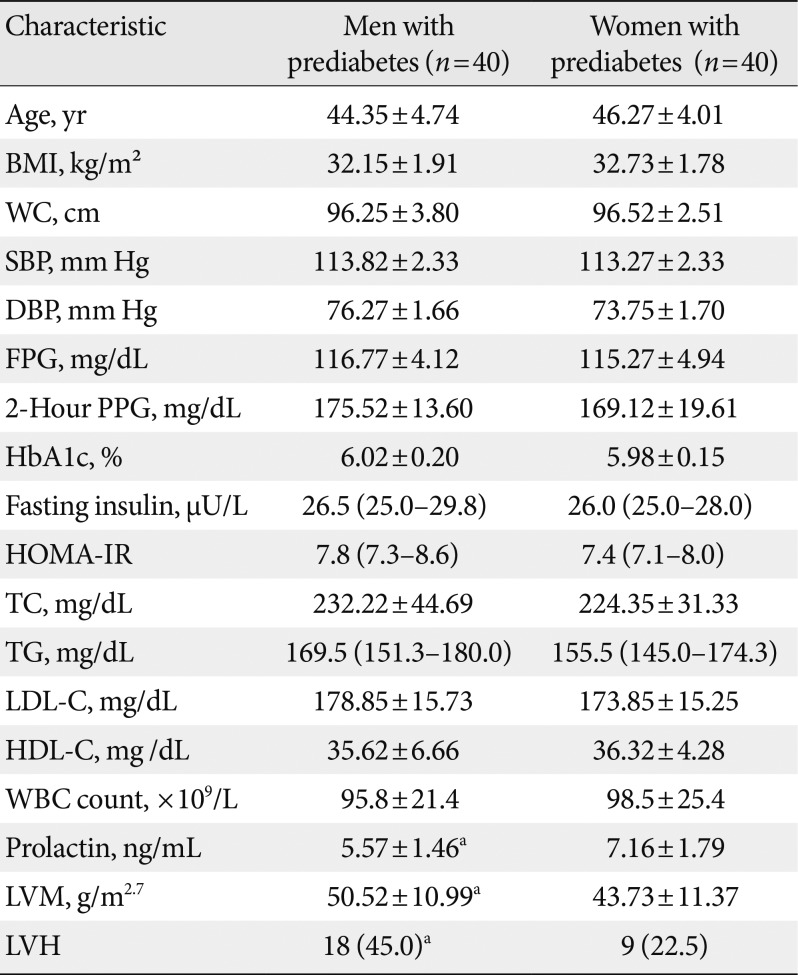
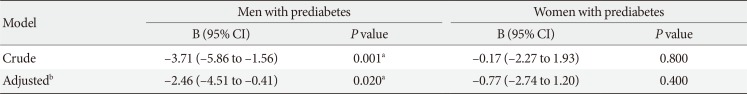
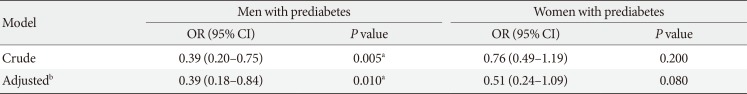
14. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–419. PMID:
3899825.
15. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502. PMID:
4337382.

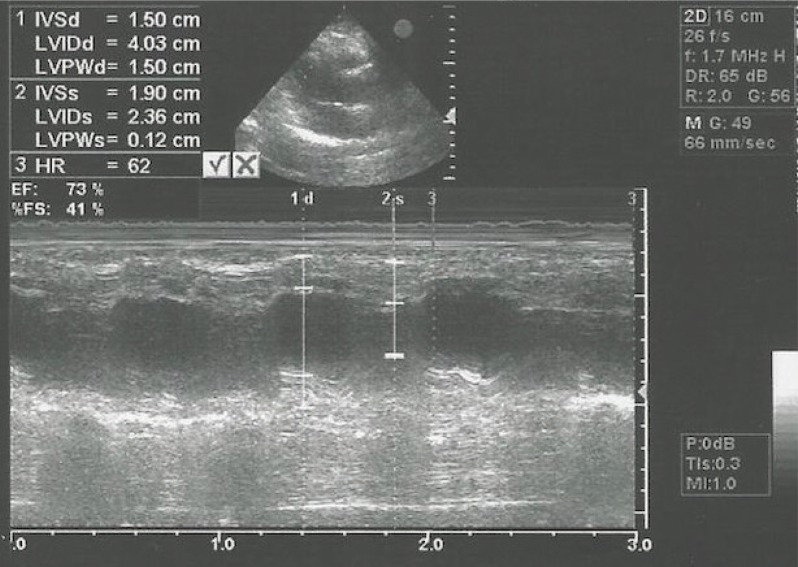
16. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Chamber Quantification Writing Group. American Society of Echocardiography's Guidelines and Standards Committee. European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440–1463. PMID:
16376782.

17. Balbach L, Wallaschofski H, Volzke H, Nauck M, Dorr M, Haring R. Serum prolactin concentrations as risk factor of metabolic syndrome or type 2 diabetes? BMC Endocr Disord. 2013; 13:12. PMID:
23517652.

18. Brelje TC, Stout LE, Bhagroo NV, Sorenson RL. Distinctive roles for prolactin and growth hormone in the activation of signal transducer and activator of transcription 5 in pancreatic islets of langerhans. Endocrinology. 2004; 145:4162–4175. PMID:
15142985.

19. Freemark M, Avril I, Fleenor D, Driscoll P, Petro A, Opara E, Kendall W, Oden J, Bridges S, Binart N, Breant B, Kelly PA. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology. 2002; 143:1378–1385. PMID:
11897695.

20. Berinder K, Nystrom T, Hoybye C, Hall K, Hulting AL. Insulin sensitivity and lipid profile in prolactinoma patients before and after normalization of prolactin by dopamine agonist therapy. Pituitary. 2011; 14:199–207. PMID:
21128120.

21. Ernst B, Thurnheer M, Schultes B. Basal serum prolactin levels in obesity: unrelated to parameters of the metabolic syndrome and unchanged after massive weight loss. Obes Surg. 2009; 19:1159–1162. PMID:
19430852.
22. Fleenor DE, Freemark M. Prolactin induction of insulin gene transcription: roles of glucose and signal transducer and activator of transcription 5. Endocrinology. 2001; 142:2805–2810. PMID:
11415999.

23. Fujinaka Y, Takane K, Yamashita H, Vasavada RC. Lactogens promote beta cell survival through JAK2/STAT5 activation and Bcl-XL upregulation. J Biol Chem. 2007; 282:30707–30717. PMID:
17728251.

24. Cejkova P, Fojtikova M, Cerna M. Immunomodulatory role of prolactin in diabetes development. Autoimmun Rev. 2009; 9:23–27. PMID:
19248843.

25. Arumugam R, Fleenor D, Lu D, Freemark M. Differential and complementary effects of glucose and prolactin on islet DNA synthesis and gene expression. Endocrinology. 2011; 152:856–868. PMID:
21239441.

26. Park S, Kang S, Lee HW, Ko BS. Central prolactin modulates insulin sensitivity and insulin secretion in diabetic rats. Neuroendocrinology. 2012; 95:332–343. PMID:
22441304.

27. Lyons DJ, Hellysaz A, Broberger C. Prolactin regulates tuberoinfundibular dopamine neuron discharge pattern: novel feedback control mechanisms in the lactotrophic axis. J Neurosci. 2012; 32:8074–8083. PMID:
22674282.

28. Ling C, Svensson L, Oden B, Weijdegard B, Eden B, Eden S, Billig H. Identification of functional prolactin (PRL) receptor gene expression: PRL inhibits lipoprotein lipase activity in human white adipose tissue. J Clin Endocrinol Metab. 2003; 88:1804–1808. PMID:
12679477.

29. Hogan JC, Stephens JM. The regulation of fatty acid synthase by STAT5A. Diabetes. 2005; 54:1968–1975. PMID:
15983196.

30. Hugo ER, Borcherding DC, Gersin KS, Loftus J, Ben-Jonathan N. Prolactin release by adipose explants, primary adipocytes, and LS14 adipocytes. J Clin Endocrinol Metab. 2008; 93:4006–4012. PMID:
18647802.

31. Brandebourg T, Hugo E, Ben-Jonathan N. Adipocyte prolactin: regulation of release and putative functions. Diabetes Obes Metab. 2007; 9:464–476. PMID:
17587388.

32. Lieb W, Xanthakis V, Sullivan LM, Aragam J, Pencina MJ, Larson MG, Benjamin EJ, Vasan RS. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short- and long-term change in the framingham offspring study. Circulation. 2009; 119:3085–3092. PMID:
19506113.
33. Corona G, Rastrelli G, Boddi V, Monami M, Melani C, Balzi D, Sforza A, Forti G, Mannucci E, Maggi M. Prolactin levels independently predict major cardiovascular events in patients with erectile dysfunction. Int J Androl. 2011; 34:217–224. PMID:
20522124.

34. Corona G, Wu FC, Rastrelli G, Lee DM, Forti G, O’Connor DB, O’Neill TW, Pendleton N, Bartfai G, Boonen S, Casanueva FF, Finn JD, Huhtaniemi IT, Kula K, Punab M, Vanderschueren D, Rutter MK, Maggi M. EMAS Study Group. Low prolactin is associated with sexual dysfunction and psychological or metabolic disturbances in middle-aged and elderly men: the European Male Aging Study (EMAS). J Sex Med. 2014; 11:240–253. PMID:
24345293.

35. Karino S, Willcox BJ, Fong K, Lo S, Abbott R, Masaki KH. Total and differential white blood cell counts predict eight-year incident coronary heart disease in elderly Japanese-American men: the Honolulu Heart Program. Atherosclerosis. 2015; 238:153–158. PMID:
25514532.

36. Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007; 128:589–600. PMID:
17289576.

37. Yamac H, Bultmann I, Sliwa K, Hilfiker-Kleiner D. Prolactin: a new therapeutic target in peripartum cardiomyopathy. Heart. 2010; 96:1352–1357. PMID:
20657009.

38. Hilfiker-Kleiner D, Hilfiker A, Drexler H. Many good reasons to have STAT3 in the heart. Pharmacol Ther. 2005; 107:131–137. PMID:
15963355.

39. Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, Hillmer A, Schmiedl A, Ding Z, Podewski E, Podewski E, Poli V, Schneider MD, Schulz R, Park JK, Wollert KC, Drexler H. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004; 95:187–195. PMID:
15192020.

40. Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, Sliwa K, Noel A, Martial JA, Hilfiker-Kleiner D, Struman I. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest. 2013; 123:2143–2154. PMID:
23619365.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download