Abstract
Non-alcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver disease in adults and children worldwide. NAFLD has become a severe health issue and it can progress towards a more severe form of the disease, the non-alcoholic steatohepatitis (NASH). A combination of environmental factors, host genetics, and gut microbiota leads to excessive accumulation of lipids in the liver (steatosis), which may result in lipotoxicity and trigger hepatocyte cell death, liver inflammation, fibrosis, and pathological angiogenesis. NASH can further progress towards liver cirrhosis and cancer. Over the last few years, cell-derived extracellular vesicles (EVs) have been identified as effective cell-to-cell messengers that transfer several bioactive molecules in target cells, modulating the pathogenesis and progression of NASH. In this review, we focused on recently highlighted aspects of molecular pathogenesis of NASH, mediated by EVs via their bioactive components. The studies included in this review summarize the state of art regarding the role of EVs during the progression of NASH and bring novel insight about the potential use of EVs for diagnosis and therapeutic strategies for patients with this disease.
All international and national health organizations report that obesity has doubled since 1982. In 2014 over 1.9 billion adults were overweight and of these over 600 million were obese worldwide. This makes obesity a serious pandemic and public health problem [123]. Importantly, childhood obesity has tripled in the last 30 years and the World Health Organization reports that 42 million children under the age of five were overweight or obese in 2013 [45]. The clinical relevance of obesity derives from the fact that obesity is associated with several metabolic risk factors that define the metabolic syndrome (MetS) [6]. MetS is strongly associated with the development of comorbidities, including cardiovascular diseases, diabetes as well as non-alcoholic fatty liver disease (NAFLD) [78910].
NAFLD is one of the most common causes of chronic liver disease (CLD) and liver-related morbidity and mortality worldwide. Currently, NAFLD has an estimated prevalence in the general population of 20% to 30% in Western Countries and 5% to 18% in Asia, and it is predicted to increase over time [11121314]. An additional dramatic increase of NAFLD has been associated with obesity and excessive fructose consumption [1516]. Notably, the overall prevalence of childhood NAFLD has reached about 10%, with a worrisome prevalence rate of 17% in teenagers [17]. This prevalence dramatically increases up to 40% to 70% among obese children [1819]. A subset of 3% to 5% of NAFLD patients can develop early non-alcoholic steatohepatitis (NASH) [2021], characterized by lobular and portal inflammatory infiltrates of monocyte-derived macrophages, neutrophils and lymphocytes; various degree of fibrosis, hepatocyte cell death and pathological angiogenesis. With the progression of the disease to advanced NASH, extensive fibrosis is associated with high risk of developing cirrhosis, liver failure, portal hypertension and/or hepatocellular carcinoma (HCC), the third leading cause of cancer death worldwide [2223]. Advanced fibrosis and cirrhosis are the principal causes of liver-related morbidity and mortality. In a recent study published by Wong and colleagues [24] on the annual trends of new liver transplant waitlist registrations they reported that from 2004 to 2013 NASH, as cause of CLD, showed an increase of almost 170% in the number of new waitlist registrations, compared to other CLDs such as alcoholic liver disease or hepatitis C virus. This makes NASH the second leading etiology of CLD in new waitlist registrations for liver transplant and projections show that NASH will become the leading cause of liver transplantation by 2020 [2425].
The most accepted concept on the pathogenesis of NAFLD involves multiple concomitant events or 'hits' associated with a crosstalk between environment, host genetics and gut microbiota [2627]. In this scenario, stressed or damaged cells release various dangerous signals that influence and drive NASH-related outcomes such as isolated steatosis, sterile inflammation, fibrosis, pathological angiogenesis, and progressive liver injury [2628]. In this review, we will focus and uncover novel insights on cell-derived extracellular vesicles (EVs) as damage-associated molecular signals involved in the molecular pathogenesis of NASH. We will describe studies reporting the EV-driven signaling and molecular pathways related to the initial and advanced events involved during the progression of NASH. We will describe the EV genesis from cells exposed to toxic agents and the reported effects on functionality and activity of the target cells.
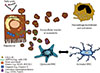
EVs (or exovesicles, as most recently named) are a heterogeneous population of small membrane-surrounded structures released by cells in the extracellular environment as well as in the bloodstream and are involved in normal physiology as well as in the pathobiology of disease [29303132]. EVs are effective communicators that are generated by a cell of origin or parenteral cell and can act on a number of target cells in an autocrine or paracrine pathway in the extracellular environment where they are released as well as in an endocrine manner, acting as long range signals [33343536]. EVs shuttle a variety of bioactive molecules that may reflect the cell of origin as well as the specific stress that induces their formation and release. They transport and transfer in target cells bioactive molecules, including mRNA, different non-coding RNAs (microRNA, longRNA mitochondrial associated tRNA, small nucleolar RNA, small nuclear RNA, Ro associated Y-RNA, vault RNA, and Y-RNA), proteins, DNA and lipids that influence and modulate various cell responses [3738]. EVs have been divided in three main categories: exosomes, ectosomes (or microparticles [MPs] or microvesicles) and apoptotic bodies (Fig. 1) [293940]. However, several reports have shown that these subpopulations have one or more characteristics in common and a clear distinction among the different categories is still not completely possible.
Exosomes are approximately 30 to 100 nm in size and originate from inward budding of the membrane of late endosoma compartments in the cytosol of cells. The fusion of endosomal compartment with the plasma membrane facilitate the release of exosomes that contain proteins, non-coding RNA (miRNA, long-RNA) and RNA interfering. Several studies have identified some exosomal membrane proteins, such as tetraspanins, CD63, CD81 and CD9 or TSG101 and Alix [3941].
Ectosomes are approximately 100 to 1,000 nm in size and are release from outward budding and blebbing of the plasma membrane directly in the extracellular space [29]. Most ectosomes express phosphatidylserine on the outer leaflet of the membrane, allowing their detection. However, a subpopulation of ectosomes does not express phosphatidylserine. For this reason different labeling approaches and cell-specific markers are used to detect this subpopulation of EVs. Ectosomes carry a variety of different bioactive molecules, such as lipid, proteins, non-coding RNA and mRNA that can be shuttled into the target cells where ectosomes are efficiently internalized and induce a variety of different cell responses [34424344].
Apoptotic bodies are the largest EVs with a diameter between 500 to 4,000 nm [45]. Similarly to ectosomes they are released by blebbing of the plasma membrane of the apoptotic cells and they express phosphatidylserine on their surface [46]. Differently from exosomes and ectosomes, apoptotic bodies carry cell organelles, DNA fragments and histones [4748]. Common markers of apoptotic bodies include complement component C3b and thrombospondin [49].
Lipotoxicity refers to a process by which accumulation of certain toxic lipids such as saturated free fatty acids (SFA), free cholesterol, or ceramide and other sphingolipid among others in hepatocytes triggers various molecular pathways of cell stress and eventually results in cell death have evolved as a key event during NAFLD progression [505152]. Elegant studies have shown that hepatocytes exposed to the SFA palmitic acid—lipotoxic lipid abundantly present in circulation of patients with NASH [5354]—produce and release large quantities of EVs that may act on various target cells and contribute to key processes involved in NAFLD pathogenesis, including immune reactions, angiogenesis, and fibrosis (Fig. 2).
Liver pathological angiogenesis consists in the development of an abnormal angioarchitecture during the progression of progressive CLDs, including metabolic-related liver diseases as NASH. The clinical relevance of pathological angiogenesis in NASH is associated with the evidence that pathological neovessel formation drives hepatic fibrogenic progression and leads to the development of cirrhosis and related complications, such as HCC [5556]. Pathological angiogenesis occurring during a CLD is primarily regulated by concomitant liver events that involved complex cell-to-cell interaction and release of various pro-angiogenic factors in the liver microenvironment [57]. Several elegant studies have reported a role of EVs in activation of endothelial cells (ECs) and promotion of angiogenesis. In our previous studies we have demonstrated that hepatocyte-derived EVs released during lipotoxicity have potent pro-angiogenic effects and promote EC activation in a Vanin-1-mediated internalization [34]. In a pioneered study by Witek et al. [58], they showed that platelet-derived growth factor BB stimulated myofibroblast-like hepatic stellate cells (HSCs) and reactive cholangiocytes, release MPs that contain active Sonic hedgehog (Shh) and Indian hedgehog (Ihh)—two of the proteins of the signaling pathway family of hedgehog. The same authors showed that plasma and bile level of Hh-containing MPs were elevated in chronic biliary fibrosis and bile duct ligation rats, respectively. MPs carrying Hh molecules induced a pro-angiogenic switch in sinusoidal ECs, followed by in vivo capillarization. In a more recent report by Lemoinne and colleagues [35], it has been demonstrated that portal myofibroblasts (PMFs) promote the formation of neo vessels triggering scarring development into the parenchyma in both CCl4-induced liver fibrosis and NASH models. Through various experimental approaches, authors showed that activated PMFs release MPs that carry and transfer the pro-angiogenic factor vascular endothelial growth factor A (VEGF-A) to ECs, inducing EC activation and tubulogenesis via VEGF-A receptor. However, the mechanism driving the release of VEGF-A by MPs was not further investigated by the authors of this published study. In conclusion, EVs play a crucial role in liver angiogenesis and may link pathological angiogenesis and fibrogenesis during the progression of NASH by transferring various agents, including growth factors, hedgehog molecules, proteins, and miRNAs.
In a recent study published by Ibrahim et al. [36], they provide novel insights in the lipotoxicity-mediated release of hepatocyte-derived EVs during NASH development. Authors report that mixed lineage kinase 3 (MLK3) mediates the release of fat-laden hepatocyte-derived EVs that carry chemokine (C-X-C motif) ligand 10 (CXCL10), a potent hepatocyte-derived macrophage chemoattractant [59]. These findings were confirmed by pharmacologically inhibiting MLK3, which resulted in a significant decrease in the amount of CXCL10 released by lipid-stressed hepatocytes. These observations highlight a novel role of CXCL10-enriched EVs in monocyte-derived macrophage recruitment and activation of resident Kupffer cells (the resident hepatic macrophages) during the progression of NASH (Fig. 3). Adipose tissue has also emerged as crucial player in the development of hepatic steatosis and chronic low-grade inflammation, a key link between obesity and the associated metabolic dysregulation [33606162]. In line with this concept, one of our recent studies first-authored by Eguchi et al. [33] shows that mature differentiated adipocytes exposed to palmitic acid resulted in the release of a marked amount of EVs that are potent chemoattractant factors that induced migration of macrophages. Through various lines of evidence, we showed that the release of EVs as chemotactic signals by adipocytes treated with palmitic acid was mediated by caspase-3 activation [3334]. These findings identified adipocyte-derived EVs as novel "find-me" signals linking adipocytes stress to macrophages recruitment. The EV-mediated cell-to-cell communication is a central event in a multifactorial disease as NASH.
Several studies have shown that advanced fibrosis and cirrhosis are crucial pathophysiological events of CLDs and lead to clinical life-threatening consequences [63646566]. Notably, advanced fibrosis has been reported to increase the risk of HCC 25-fold [2367]. Activated HSCs are the main cell type that induce and sustain the extracellular matrix remodeling and collagen deposition during liver fibrogenesis [68697071]. Among many factors, circulating miRNAs are pivotal regulators of HSC phenotype and several studies have identified miRNAs up-regulated in experimental models of NAFLD or activated HSC in vitro [72737475]. For an extensive and complete overview on the role of miRNAs in the pathogenesis of CLDs we recommend other reviews [7677787980]. In this paragraph we will highlight some studies describing the role of EVs in liver fibrogenesis in NASH. A first study showing that HSCs can be activated by EVs was published by Kornek and colleagues [81]. Authors described the role of T cells in the fibrolytic activation of HSCs [81]. In this study it was demonstrated that activated and apoptotic CD4+ and CD8+ T cells release MPs in the extracellular environment and in circulation that fuse with HSCs and transfer membrane molecules such as CD147, triggering the upregulation of fibrolytic molecules. The biological significance of "horizontal transfer" of bioactive molecules by EVs has been demonstrated by various studies. Pro-fibrogenic connective tissue growth factor (CCN2) has been identified exosomes packaged by activated HSCs and shuttled to other quiescent or activated HSCs as form of paracrine pro-fibrogenic activation during a liver injury [82]. On the other hand, a second study showed that exosomes released by quiescent HSCs have an anti-fibrogenic potential by transferring Twist1, a transcription factor that activates miR-214 and results in CCN2 suppression [83]. The evidence that EVs can shuttle and deliver small or long non-coding RNA has sparked a great interest in the basic pathological mechanisms of liver fibrosis development. Notably, it has been demonstrated that EVs shred by hepatocytes exposed to palmitic acid shuttle miR-128-3p into HSCs and induce a down-regulation of PPAR-gamma, a central modulator of HSC quiescence (Fig. 3) [84858687]. In a complex microenvironment such as the one present during NASH-related fibrogenesis, EVs released by several cell sources play a key role in the homeostasis and balance between pro-fibrogenic and anti-fibrogenic mechanisms.
Currently, liver biopsy remains the gold standard procedure to distinguish simple steatosis from steatohepatitis. However, it is costly, invasive and carries some morbidity and a rare mortality risk [63]. The biogenesis and composition of EVs have suggested that they might be identified as novel non-invasive and reliable biomarkers for liver injury during NASH development. This concept is further supported by the evidence that EVs can be detected and isolated from a variety of biofluids, including serum and plasma samples both from experimental animal models and human patients with NASH [888990]. Level of circulating MP derived from CD14+ and invariant natural killer T (iNKT) cells were significantly elevated in patients with NAFLD/NASH, independently of alanine aminotransferase levels or histopathological markers of disease activity [91]. In line with these findings, a complete characterization of bioavailability, composition, and origin of circulating EVs would indicate whether EVs can be used to discriminate different CLDs or different disease stages during the progression of CLDs such as NASH. Experimental evidence supporting this concept has been shown in one of our recent reports. In an experimental model of diet-induced NAFLD, early- and advanced-NASH, we observed that level of blood EVs increased over time during the NASH progression and was strongly associated with some key outcomes of NASH, including the extent of liver fibrosis, angiogenesis, and cell death [92]. The bioavailability of EVs in biofluids is only one of the fascinating aspects of these effective "messengers." Indeed, the composition of EVs suggests an extraordinary potentiality for biomarker development. As a proof of this, miRNAs encapsulated in circulating EVs have increasingly exhibited great potential to fulfill several criteria for being considered a good biomarker, such as specificity, sensitivity, robustness, predictivity, translatability, and non-invasiveness [909394]. Circulating EVs isolated from murine models of experimental NASH were enriched in miR-122 and miR-192, two microRNAs abundantly expressed in the liver [9596]. The level of these microRNAs increased in EVs and decreased in livers over time during NAFLD progression [92]. The release of these microRNAs from stressed or damaged hepatocytes in EVs during NAFLD progression may provide an attractive explanation for the decreased expression level of miR-122 found in the livers of patients with advanced NAFLD [97], as well as early stage hepatocarcinogenesis from NASH in both an animal model and human tissue samples, as recently reported [9899]. In addition to microRNAs, a complete proteomics analysis of circulating EVs isolated from rodents with NASH vs. controls revealed a unique disease-specific protein signature that can be employed as "barcode" for diagnostic novel strategies in NASH [92]. These findings provide important support to the development of EV-based lab-on-a-chip devices [100] that can be used for "liquid biopsy" to introduce novel and non-invasive diagnostic strategies for NASH.
In conclusion, we have highlighted several of the most recent and original studies reporting the role of EVs as link between lipotoxicity, inflammation, angiogenesis, and fibrosis, all key events in the pathogenesis of NASH. Evidence shows that EVs may act as or transfer damage-associated molecular patterns to target cells of the liver microenvironment and orchestrate many of the main pathological events occurring concurrently during the development of NASH. A second important aspect of EVs consists on the fate of EVs, either benign or malignant and this seems to depend on the phenotype of the cell of origin.
Future studies will need to further (1) dissect the mechanisms of release of EVs by the parenteral cells in pathological conditions; (2) investigate additional functions of EVs during the pathogenesis of NASH; and (3) elucidate whether an impaired balance between "bad" and "good" EVs might be a potential cause of many CLDs.
Figures and Tables
Fig. 1
Extracellular vesicles at play. Extracellular vesicles (EVs) or exovesicles are membrane surrounded structures release by cells both during physiological as well as under various stress conditions and they can be divided into exosomes, ectosomes, or microparticles, and apoptotic bodies. Exosomes (30 to 100 nm) are released via exocytosis through the fusion of multivesicular bodies (MVB) with plasma membranes. Ectosomes are vesicles 100 to 1,000 nm in size and they are released by direct blebbing/budding from the plasma membrane. Apoptotic bodies are released by fragmentation of a cell during programmed cell death. Exovesicles express several cell specific and stress specific markers and carry a variety of bioactive molecules, including non-coding long and micro-RNAs, mRNA, lipids, and proteins. TSG101, tumor susceptibility gene 101.
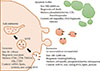
Fig. 2
Extracellular vesicles in the pathogenesis of fatty liver disease. During the process of lipotoxicity, hepatocytes release large quantities of extracellular vesicles (EVs) that may then act on various target cells in the local environment they are released contributing to key processes involved in non-alcoholic fatty liver disease pathogenesis including immune modulation, angiogenesis, and fibrosis. Additionally, EVs may be released to the systemic circulation and can be potentially used to non-invasively monitoring the extent of liver injury.
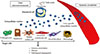
Fig. 3
Hepatocyte-derived extracellular vesicles released during lipotoxicity and their role in liver fibrosis and inflammation. Lipid-induced toxicity (lipotoxicity) due to over-accumulation of various toxic lipids in hepatocytes such as saturated free fatty acids induce the release of exovesicles in a process that may involved activation of caspases such as caspase-3 as well as stress-activated kinases such MLK3, ROCK1. Liver-derived exovesicles carry several bioactive molecules (e.g., CXCL10, connective tissue growth factor, Twist1) and non-coding small or long RNAs, such as miR-128. These signals are shuttled by exovesicles and can modulate inflammation and fibrogenesis by recruiting or activating macrophages and quiescent hepatic stellate cells (HSCs), respectively. Notably, anti-fibrotic factors can be also transferred into extracellular vesicles and lead to HSC inactivation. CXCL10, chemokine (C-X-C motif) ligand 10; MLK3, mixed lineage kinase 3; ROCK, Rho-associated coiled-coil-containing protein kinase 1; CCN, connective tissue growth factor.
ACKNOWLEDGMENTS
This work was partially supported by NIH grants U01 AA022489, R21 AA023574 to A.E.F. and Roger L. Jenkins American Liver Foundation Postdoctoral Research Fellowship to D.P. We kindly thank Prof. Massimo Pinzani for the use of his hepatic stellate cell image.
References
1. Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. JAMA. 2014; 312:189–190.
2. Kang HT, Shim JY, Lee HR, Park BJ, Linton JA, Lee YJ. Trends in prevalence of overweight and obesity in Korean adults, 1998-2009: the Korean National Health and Nutrition Examination Survey. J Epidemiol. 2014; 24:109–116.
3. Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991-1998. JAMA. 1999; 282:1519–1522.
4. Lasserre AM, Chiolero A, Paccaud F, Bovet P. Worldwide trends in childhood obesity. Swiss Med Wkly. 2007; 137:157–158.
5. Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006; 1:11–25.
6. Katzmarzyk PT, Church TS, Janssen I, Ross R, Blair SN. Metabolic syndrome, obesity, and mortality: impact of cardiorespiratory fitness. Diabetes Care. 2005; 28:391–397.
7. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. American Heart Association. Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006; 113:898–918.
8. Tiehuis AM, van der Graaf Y, Mali WP, Vincken K, Muller M, Geerlings MI. SMART Study Group. Metabolic syndrome, prediabetes, and brain abnormalities on mri in patients with manifest arterial disease: the SMART-MR study. Diabetes Care. 2014; 37:2515–2521.
9. Kumar PA, Chitra PS, Reddy GB. Metabolic syndrome and associated chronic kidney diseases: nutritional interventions. Rev Endocr Metab Disord. 2013; 14:273–286.
10. Kwon YM, Oh SW, Hwang SS, Lee C, Kwon H, Chung GE. Association of nonalcoholic fatty liver disease with components of metabolic syndrome according to body mass index in Korean adults. Am J Gastroenterol. 2012; 107:1852–1858.
11. Masarone M, Federico A, Abenavoli L, Loguercio C, Persico M. Non alcoholic fatty liver: epidemiology and natural history. Rev Recent Clin Trials. 2014; 9:126–133.
12. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013; 10:686–690.
13. Brunt EM. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010; 7:195–203.
14. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005; 129:113–121.
15. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010; 51:679–689.
16. Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008; 48:993–999.
17. Patton HM, Sirlin C, Behling C, Middleton M, Schwimmer JB, Lavine JE. Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. J Pediatr Gastroenterol Nutr. 2006; 43:413–427.
18. Schwimmer JB, Newton KP, Awai HI, Choi LJ, Garcia MA, Ellis LL, Vanderwall K, Fontanesi J. Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013; 38:1267–1277.
19. Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008; 118:277–283.
20. Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011; 140:124–131.
21. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011; 34:274–285.
22. Sorensen HT, Mellemkjaer L, Jepsen P, Thulstrup AM, Baron J, Olsen JH, Vilstrup H. Risk of cancer in patients hospitalized with fatty liver: a Danish cohort study. J Clin Gastroenterol. 2003; 36:356–359.
23. Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010; 51:1972–1978.
24. Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015; 148:547–555.
25. Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013; 368:1859–1861.
26. Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010; 52:1836–1846.
27. Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012; 56:952–964.
28. Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 2013; 14:20704–20728.
29. Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014; 30:255–289.
30. Lemoinne S, Thabut D, Housset C, Moreau R, Valla D, Boulanger CM, Rautou PE. The emerging roles of microvesicles in liver diseases. Nat Rev Gastroenterol Hepatol. 2014; 11:350–361.
31. Thery C. Cancer: diagnosis by extracellular vesicles. Nature. 2015; 523:161–162.
32. Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015; 4:27066.
33. Eguchi A, Mulya A, Lazic M, Radhakrishnan D, Berk MP, Povero D, Gornicka A, Feldstein AE. Microparticles release by adipocytes act as "find-me" signals to promote macrophage migration. PLoS One. 2015; 10:e0123110.
34. Povero D, Eguchi A, Niesman IR, Andronikou N, de Mollerat du Jeu X, Mulya A, Berk M, Lazic M, Thapaliya S, Parola M, Patel HH, Feldstein AE. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci Signal. 2013; 6:ra88.
35. Lemoinne S, Cadoret A, Rautou PE, El Mourabit H, Ratziu V, Corpechot C, Rey C, Bosselut N, Barbu V, Wendum D, Feldmann G, Boulanger C, Henegar C, Housset C, Thabut D. Portal myofibroblasts promote vascular remodeling underlying cirrhosis formation through the release of microparticles. Hepatology. 2015; 61:1041–1055.
36. Ibrahim SH, Hirsova P, Tomita K, Bronk SF, Werneburg NW, Harrison SA, Goodfellow VS, Malhi H, Gores GJ. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2015; 09. 25. [Epub]. DOI: 10.1002/hep.28252.
37. Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, Buzas EI, Lotvall J. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013; 2.
38. Lunavat TR, Cheng L, Kim DK, Bhadury J, Jang SC, Lasser C, Sharples RA, Lopez MD, Nilsson J, Gho YS, Hill AF, Lotvall J. Small RNA deep sequencing discriminates subsets of extracellular vesicles released by melanoma cells: evidence of unique microRNA cargos. RNA Biol. 2015; 12:810–823.
39. Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A, Buzas EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011; 68:2667–2688.
40. EL Andaloussi S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013; 12:347–357.
41. Keerthikumar S, Gangoda L, Liem M, Fonseka P, Atukorala I, Ozcitti C, Mechler A, Adda CG, Ang CS, Mathivanan S. Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes. Oncotarget. 2015; 6:15375–15396.
42. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013; 200:373–383.
43. Cocucci E, Meldolesi J. Ectosomes. Curr Biol. 2011; 21:R940–R941.
44. O'Driscoll L. Expanding on exosomes and ectosomes in cancer. N Engl J Med. 2015; 372:2359–2362.
45. Depraetere V. "Eat me" signals of apoptotic bodies. Nat Cell Biol. 2000; 2:E104.
46. Catchpoole DR, Stewart BW. Formation of apoptotic bodies is associated with internucleosomal DNA fragmentation during drug-induced apoptosis. Exp Cell Res. 1995; 216:169–177.
47. Lleo A, Zhang W, McDonald WH, Seeley EH, Leung PS, Coppel RL, Ansari AA, Adams DH, Afford S, Invernizzi P, Gershwin ME. Shotgun proteomics: identification of unique protein profiles of apoptotic bodies from biliary epithelial cells. Hepatology. 2014; 60:1314–1323.
48. Halicka HD, Bedner E, Darzynkiewicz Z. Segregation of RNA and separate packaging of DNA and RNA in apoptotic bodies during apoptosis. Exp Cell Res. 2000; 260:248–256.
49. Revenfeld AL, Baek R, Nielsen MH, Stensballe A, Varming K, Jorgensen M. Diagnostic and prognostic potential of extracellular vesicles in peripheral blood. Clin Ther. 2014; 36:830–846.
50. Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009; 3:445–451.
51. Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004; 40:185–194.
52. Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008; 47:1495–1503.
53. Cheung O, Kapoor A, Puri P, Sistrun S, Luketic VA, Sargeant CC, Contos MJ, Shiffman ML, Stravitz RT, Sterling RK, Sanyal AJ. The impact of fat distribution on the severity of nonalcoholic fatty liver disease and metabolic syndrome. Hepatology. 2007; 46:1091–1100.
54. Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007; 46:1081–1090.
55. Weston CJ, Shepherd EL, Claridge LC, Rantakari P, Curbishley SM, Tomlinson JW, Hubscher SG, Reynolds GM, Aalto K, Anstee QM, Jalkanen S, Salmi M, Smith DJ, Day CP, Adams DH. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis. J Clin Invest. 2015; 125:501–520.
56. Valfre di Bonzo L, Novo E, Cannito S, Busletta C, Paternostro C, Povero D, Parola M. Angiogenesis and liver fibrogenesis. Histol Histopathol. 2009; 24:1323–1341.
57. Bocca C, Novo E, Miglietta A, Parola M. Angiogenesis and fibrogenesis in chronic liver diseases. Cell Mol Gastroenterol Hepatol. 2015; 1:477–488.
58. Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, Choi SS, Cheong Y, Fearing CM, Agboola KM, Chen W, Diehl AM. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009; 136:320–330.e2.
59. Fahey S, Dempsey E, Long A. The role of chemokines in acute and chronic hepatitis C infection. Cell Mol Immunol. 2014; 11:25–40.
60. Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008; 14:222–231.
61. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–1808.
62. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007; 117:175–184.
63. Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L. American Association for the Study of Liver Diseases. United States Food and Drug Administration. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology. 2015; 61:1392–1405.
64. Taouli B, Ehman RL, Reeder SB. Advanced MRI methods for assessment of chronic liver disease. AJR Am J Roentgenol. 2009; 193:14–27.
65. D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006; 44:217–231.
66. Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005; 115:209–218.
67. Sorensen HT, Friis S, Norgard B, Mellemkjaer L, Blot WJ, McLaughlin JK, Ekbom A, Baron JA. Risk of cancer in a large cohort of nonaspirin NSAID users: a population-based study. Br J Cancer. 2003; 88:1687–1692.
68. Schuppan D, Pinzani M. Anti-fibrotic therapy: lost in translation? J Hepatol. 2012; 56:Suppl 1. S66–S74.
69. Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001; 21:437–451.
70. Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008; 134:1655–1669.
71. Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015; 64:830–841.
72. Guo CJ, Pan Q, Xiong H, Qiao YQ, Bian ZL, Zhong W, Sheng L, Li H, Shen L, Hua J, Ma X, Fang JY. Dynamic expression of miR-126* and its effects on proliferation and contraction of hepatic stellate cells. FEBS Lett. 2013; 587:3792–3801.
73. Venugopal SK, Jiang J, Kim TH, Li Y, Wang SS, Torok NJ, Wu J, Zern MA. Liver fibrosis causes downregulation of miRNA-150 and miRNA-194 in hepatic stellate cells, and their overexpression causes decreased stellate cell activation. Am J Physiol Gastrointest Liver Physiol. 2010; 298:G101–G106.
74. He Y, Huang C, Zhang SP, Sun X, Long XR, Li J. The potential of microRNAs in liver fibrosis. Cell Signal. 2012; 24:2268–2272.
75. Zheng J, Wu C, Xu Z, Xia P, Dong P, Chen B, Yu F. Hepatic stellate cell is activated by microRNA-181b via PTEN/Akt pathway. Mol Cell Biochem. 2015; 398:1–9.
76. Arrese M, Eguchi A, Feldstein AE. Circulating microRNAs: emerging biomarkers of liver disease. Semin Liver Dis. 2015; 35:43–54.
77. Wang Y, Chen T, Tong W. miRNAs and their application in drug-induced liver injury. Biomark Med. 2014; 8:161–172.
78. Wang XW, Heegaard NH, Orum H. MicroRNAs in liver disease. Gastroenterology. 2012; 142:1431–1443.
79. Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013; 10:542–552.
80. Pirola CJ, Fernandez Gianotti T, Castano GO, Mallardi P, San Martino J, Mora Gonzalez Lopez Ledesma M, Flichman D, Mirshahi F, Sanyal AJ, Sookoian S. Circulating microRNA signature in non-alcoholic fatty liver disease: from serum non-coding RNAs to liver histology and disease pathogenesis. Gut. 2015; 64:800–812.
81. Kornek M, Popov Y, Libermann TA, Afdhal NH, Schuppan D. Human T cell microparticles circulate in blood of hepatitis patients and induce fibrolytic activation of hepatic stellate cells. Hepatology. 2011; 53:230–242.
82. Charrier A, Chen R, Chen L, Kemper S, Hattori T, Takigawa M, Brigstock DR. Exosomes mediate intercellular transfer of pro-fibrogenic connective tissue growth factor (CCN2) between hepatic stellate cells, the principal fibrotic cells in the liver. Surgery. 2014; 156:548–555.
83. Chen L, Chen R, Kemper S, Charrier A, Brigstock DR. Suppression of fibrogenic signaling in hepatic stellate cells by Twist1-dependent microRNA-214 expression: role of exosomes in horizontal transfer of Twist1. Am J Physiol Gastrointest Liver Physiol. 2015; 309:G491–G499.
84. Hazra S, Miyahara T, Rippe RA, Tsukamoto H. PPAR gamma and hepatic stellate cells. Comp Hepatol. 2004; 3:Suppl 1. S7.
85. Hazra S, Xiong S, Wang J, Rippe RA, Krishna V, Chatterjee K, Tsukamoto H. Peroxisome proliferator-activated receptor gamma induces a phenotypic switch from activated to quiescent hepatic stellate cells. J Biol Chem. 2004; 279:11392–11401.
86. Povero D, Panera N, Eguchi A, Johnson CD, Papouchado BG, de Araujo Horcel L, Pinatel EM, Alisi A, Nobili V, Feldstein AE. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cells via microRNA targeting peroxisome proliferator-activated receptor-γ. Cell Mol Gastroenterol Hepatol. 2015; 1:646–663.e4.
87. Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, Batignani G, Bonacchi A, Caporale R, Laffi G, Pinzani M, Gentilini P. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000; 119:466–478.
88. Akers JC, Ramakrishnan V, Kim R, Phillips S, Kaimal V, Mao Y, Hua W, Yang I, Fu CC, Nolan J, Nakano I, Yang Y, Beaulieu M, Carter BS, Chen CC. miRNA contents of cerebrospinal fluid extracellular vesicles in glioblastoma patients. J Neurooncol. 2015; 123:205–216.
89. Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire CA, Chen JW, Tannous BA, Breakefield XO. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014; 8:483–494.
90. Santiago-Dieppa DR, Steinberg J, Gonda D, Cheung VJ, Carter BS, Chen CC. Extracellular vesicles as a platform for 'liquid biopsy' in glioblastoma patients. Expert Rev Mol Diagn. 2014; 14:819–825.
91. Kornek M, Lynch M, Mehta SH, Lai M, Exley M, Afdhal NH, Schuppan D. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology. 2012; 143:448–458.
92. Povero D, Eguchi A, Li H, Johnson CD, Papouchado BG, Wree A, Messer K, Feldstein AE. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS One. 2014; 9:e113651.
93. Momen-Heravi F, Saha B, Kodys K, Catalano D, Satishchandran A, Szabo G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J Transl Med. 2015; 13:261.
94. Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014; 3.
95. Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, Alao H, Kodys K, Szabo G. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012; 56:1946–1957.
96. Csak T, Bala S, Lippai D, Satishchandran A, Catalano D, Kodys K, Szabo G. microRNA-122 regulates hypoxia-inducible factor-1 and vimentin in hepatocytes and correlates with fibrosis in diet-induced steatohepatitis. Liver Int. 2015; 35:532–541.
97. Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, Kellum JM, Min H, Luketic VA, Sanyal AJ. Nonalcoholic steatohepatitis is associated with altered hepatic microRNA expression. Hepatology. 2008; 48:1810–1820.
98. Takaki Y, Saito Y, Takasugi A, Toshimitsu K, Yamada S, Muramatsu T, Kimura M, Sugiyama K, Suzuki H, Arai E, Ojima H, Kanai Y, Saito H. Silencing of microRNA-122 is an early event during hepatocarcinogenesis from non-alcoholic steatohepatitis. Cancer Sci. 2014; 105:1254–1260.
99. Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, Subramaniam A, Propp S, Lollo BA, Freier S, Bennett CF, Bhanot S, Monia BP. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006; 3:87–98.
100. Yeo LY, Chang HC, Chan PP, Friend JR. Microfluidic devices for bioapplications. Small. 2011; 7:12–48.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download