Abstract
Diabetic nephropathy is a leading cause of end stage renal disease and its occurance is increasing worldwide. The most effective treatment strategy for the condition is intensive treatment to strictly control glycemia and blood pressure using renin-angiotensin system inhibitors. However, a fraction of patients still go on to reach end stage renal disease even under such intensive care. New therapeutic targets for diabetic nephropathy are, therefore, urgently needed. Autophagy is a major catabolic pathway by which mammalian cells degrade macromolecules and organelles to maintain intracellular homeostasis. The accumulation of damaged proteins and organelles is associated with the pathogenesis of diabetic nephropathy. Autophagy in the kidney is activated under some stress conditions, such as oxidative stress and hypoxia in proximal tubular cells, and occurs even under normal conditions in podocytes. These and other accumulating findings have led to a hypothesis that autophagy is involved in the pathogenesis of diabetic nephropathy. Here, we review recent findings underpinning this hypothesis and discuss the advantages of targeting autophagy for the treatment of diabetic nephropathy.
Diabetic nephropathy is a leading cause of end-stage renal disease throughout the world. The establishment of novel, effective therapeutic strategies is, therefore, urgently required. Proteinuria and/or albuminuria is a sign of glomerular lesions in diabetic nephropathy. These lesions can subsequently develop into tubulointerstitial lesions that lead to renal dysfunction [1]. Clinically, therefore, reducing proteinuria is considered a principal therapeutic target to improve renal outcomes in patients with diabetic nephropathy.
The pathogenesis of diabetic nephropathy involves altered intracellular metabolism associated with hyperglycemia, including the activation of protein kinase C, the accumulation of advanced glycation end-products, increased flux of the polyol pathway and oxidative stress [23456]. Moreover, hemodynamic changes such as systemic and glomerular hypertension related to hyperactivation of the renin-angiotensin system are involved in diabetic nephropathy [7]. The strong association of these alterations with the pathogenesis of diabetic nephropathy has been supported by a number of large clinical trials such as the Diabetes Control and Complications Trial (DCCT), United Kingdom Prospective Diabetes Study (UKPDS), Kumamoto study and Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENNAL) study, all of which showed that intensive control of glycemia and blood pressure with the blockades of renin-angiotensin system could successfully prevent the progression of diabetic nephropathy [78910]. Furthermore, interestingly, recent clinical studies have revealed that microalbuminuria was reversible and overt proteinuria partially reversible with strict glycemic and blood pressure controls [1112]. Thus, some aspects of diabetic nephropathy are becoming treatable. However, it is also an undeniable fact that some patients develop treatment-resistant proteinuria, such as nephrotic syndrome, resulting in end stage renal disease. Thus, additional therapeutic options are needed to further reduce proteinuria and/or to protect proximal tubular cells from proteinuria-related toxicity.
Compared with several proteinuric kidney diseases, the renal prognosis of patients with diabetic nephropathy is extremely poor. This suggests that the diabetic condition makes various renal cells vulnerable to damage. Cells have evolved several mechanisms to cope with stress and to maintain cellular homeostasis, such as the anti-oxidative stress response [5] and the endoplasmic reticulum (ER) stress response [13]. In addition, autophagy is an intracellular catabolic processes, in which proteins and organelles are degraded via lysosomes to maintain intracellular homeostasis under certain cytotoxic stress conditions, including hypoxia and ER stress [14]. Recent reports have shown that autophagy activity declines with obesity or aging in some organs, a decline that is associated with the pathogenesis of obesity- and age-related diseases [1516]. The functional roles of autophagy in the kidney have been intensely investigated [17]. For example, autophagy has been reported to play renoprotective roles during both normal aging and after acute kidney injury in some animal models [1819]. These findings allow us to hypothesize that diabetes can impair autophagic activity, making kidney cells vulnerable to diabetes-related metabolic stress and to consider autophagy as a new therapeutic target for diabetic nephropathy [20]. In this review, we summarize recent experimental findings regarding the role of autophagy in diabetic nephropathy and discuss its therapeutic potential.
The term "autophagy" is derived from Greek, and means self-eating. It is a bulk degradation process involved in the clearance of damaged proteins and organelles and is a highly conserved process from yeast to mammals [21]. Autophagy has two major physiological roles in cells. One is to recycle intracellular energy resources in response to conditions of nutrient depletion [22], and the other is to remove cytotoxic proteins and damaged organelles under various stress conditions [14]. Thus, autophagy is recognized as an essential system to maintain cellular homeostasis. Several types of autophagy have been described, including macroautophagy, microautophagy, and chaperone-mediated autophagy, all of which differ in their mechanisms and functions. Of these three types, macroautophagy is the most prevalent and is hereafter referred to as autophagy.
During autophagy, de novo isolation membranes (phagophores) elongate and fuse while engulfing a portion of the cytoplasm within double-membraned vesicles (autophagosomes) [21]. Autophagosomes can originate from the ER membranes. Four major steps are involved in the formation of autophago-somes: initiation, nucleation, elongation, and closure [21], each of which is strictly regulated by proteins encoded by autophagy-related genes (Atgs).
Autophagy is initiated by activation of the unc-51-like kinase 1 (Ulk1) complex, the mammalian ortholog of the yeast Atg1 complex. The Ulk1 complex is composed of the Ulk1 Ser/Thr protein kinase, Atg13 and FIP200, the mammalian homolog of the yeast Atg17 protein. Ulk1-mediated phosphorylation of Atg13 and FIP200 is essential for triggering autophagy. Phago-phore nucleation is dependent on a Beclin1 (Atg6 in yeast), hVps34 complex, or class III phosphatidylinositol 3-kinase (PI3K) complex, which consists of Beclin1, hVps34, hVps15, and Atg14 [2324]. Autophagosome elongation/closure involves two ubiquitin-like conjugation systems: Atg12 and light chain 3 (LC3), the mammalian ortholog of yeast Atg8. The Atg12-Atg5 conjugate, which forms the Atg12-Atg5-Atg16 complex, contributes to the stimulation and localization of the LC3 conjugation reaction. The cytosolic isoform of LC3 (LC3-I) is conjugated to phosphatidylethanolamine through two consecutive ubiquitination-like reactions catalyzed by the E1-like enzyme Atg7 and the E2-like enzyme Atg3, forming LC3-II. Thus, LC3-II formation is recognized as a marker for autophagosomes in cell and animal experiments [21]. After formation, the autophagosomes merge with lysosomal compartments to form autolysosomes. Finally, autophagosome containing macromolecules can be degraded by lysosome enzymes. The protein p62, also known as sequestosome 1 (SQSTM1), localizes to autophagosomes by interacting with LC3 and is consistently degraded by the autophagy-lysosome system [25]. The accumulation of p62 has been observed in autophagy-deficient cells [25].
Autophagy is triggered by nutrient starvation conditions and functions to overcome such a life-threatening situation. Thus, the signaling associated with autophagy is mostly understood in the context of coping with nutritional stress and maintaining cellular homeostasis. Many studies have concentrated on amino acid and insulin-dependent signaling involving mammalian target of rapamycin complex 1 (mTORC1). Hyperactivation of mTORC1 due to amino acid load or insulin stimulation can inhibit autophagy at the step of autophagy initiation [26]. Upstream of mTORC1, 5'-adenosine monophosphate (AMP)-activated protein kinase (AMPK) phosphorylates and activates tuberous sclerosis 2, an inhibitor of mTORC1, in response to a low adenosine triphosphate/AMP ratio, leading to mTORC1 inactivation and autophagy induction [27]. Furthermore, under glucose starvation, AMPK promotes autophagy by directly activating Ulk1 through phosphorylation of Ser317 and Ser777 [28]. In contrast, under nutrient sufficiency, high mTORC1 activity prevents Ulk1 activation by phosphorylating Ulk1 Ser757 and disrupting the interaction between Ulk1 and AMPK [28]. This coordinated phosphorylation is important for Ulk1 in autophagy induction. Thus, mTORC1 and AMPK play central and opposite roles in regulating the initiation of autophagy in response to alterations in intra- and extra-cellular nutrient levels (Fig. 1).
Sirt1 is a nicotinamide adenine dinucleotide (NAD)-dependent deacetylase that can also act as an intracellular nutrient sensing signal through its deacetylase activity on some tran scriptional factors and cytosolic proteins [29]. Sirt1 is activated under energy depleted conditions in response to an increase in the ratio of NAD/NADH. Sirt1 can directly deacetylate some Atg proteins, such as Atg5, Atg7, and Atg8, and this process is required for starvation-induced autophagy activation [30]. Furthermore, Sirt1 deacetylates forkhead box O3a (FOXO3a), which results in upregulation of Bnip3 gene transcription, resulting in Beclin-1-dependent initiation of autophagy [19]. Thus, Sirt1 regulates autophagosome formation via multiple components of the autophagy machinery (Fig. 1).
As mentioned above, once autophagy is activated, LC3 proteins localize to the autophagosome membrane. This can be observed in a transgenic mouse carrying a green fluorescent protein (GFP)-LC3 fusion transgene as a green dot signal and, therefore, activation of autophagy can be detected in cells of the transgenic mouse [31]. This animal model is widely employed and we used it to examine autophagy activity in kidneys. As previously reported [31], podocytes show active autophagy regardless of feeding condition (Fig. 2). In contrast, proximal tubular cells show autophagy activation only when they were exposed to starvation (Fig. 2). Using the GFP-LC3 mouse model, we did not find autophagosome dots in renal cells other than podocytes and proximal tubular cells, for example in cells of the distal tubules and collecting duct. These results are consistent with recent reports from other investigators. Thus, podocytes and proximal tubular cells are the focus of autophagy studies in the field of kidney research.
Proteinuria is a major clinical concern of diabetic nephropathy and is caused by disruption to the glomerular filtration barrier. Glomerular epithelial cells, also called podocytes, are predominantly responsible for maintaining the glomerular filtration barrier [32]. Podocytes are highly specialized, terminally differentiated and unable to proliferate. Podocyte loss due to cell death and podocyte foot process dysfunction result in massive proteinuria in diabetic nephropathy [33]. Thus, maintaining podocyte cell homeostasis is regarded as a therapeutic target to prevent progression to nephrotic syndrome due to diabetic nephropathy.
The autophagy-lysosomal degradation pathway is likely to play an essential role in maintaining podocyte function. As mentioned above, podocytes exhibit active autophagy even under non-stress conditions, suggesting that podocytes require a high basal level of autophagy to maintain cellular homeostasis [31]. Podocyte-specific autophagy-deficient mice, resulting from Atg5 gene deletion, have glomerular lesions accompanied by podocyte loss and albuminuria that increase with age [18]. Furthermore, the impairment of lysosomal function in podocytes by deletion of the mammalian target of rapamycin (mTOR), prorenin receptor or mVps34 genes caused severe glomerular sclerosis, massive proteinuria [343536]. Since autophagy involves degradation by lysosomes, autophagosomal degradation was disturbed in the podocytes of these mouse models. These results support the idea that the autophagy-lysosomal degradation pathway plays an essential role in maintaining podocyte cell homeostasis.
Despite this knowledge of the physiological role of podocyte autophagy, the role of autophagy in diabetic nephropathy has remained unclear. However, accumulating evidence now shows an association between autophagy and diabetic nephropathy. Autophagic activity in podocytes of streptozotocin-induced diabetic mice was found to decline with increased duration of diabetes [37]. Cultured podocytes exposed to high concentrations of glucose also showed lower autophagic activity, along with decreased levels of autophagy-related proteins, such as beclin-1 and the Atg5-Atg12 complex [37]. Furthermore, suppression of beclin-1 expression in cultured podocytes decreased podocin expression resulting in albumin leakage [37]. These findings suggest that hyperglycemia reduces autophagy activity, leading to alterations in podocyte function and disturbance to the glomerular filtration barrier.
Although many of these results support the hypothesis that autophagy is involved in the pathogenesis of diabetic nephropathy, direct evidence showing the involvement of autophagy in the pathogenesis of diabetic nephropathy using podocyte-specific autophagy deficient animals had not been reported until recently. However, two recent works, including our own, have clearly shown the renoprotective role of autophagy in diabetic podocytes [3839]. Podocyte-specific autophagy-deficient mice developed podocyte loss and massive proteinuria when used in a high-fat diet (HFD)-induced obese type 2 diabetic model [38]. Furthermore, deletion of Atg5 specifically in podocytes resulted in accelerated diabetes-induced podocytopathy with a leaky glomerular filtration barrier in a streptozotocin-induced type 1 diabetic model [39]. Collectively, these findings suggest that autophagy is likely to play an essential role in coping with diabetic stress in podocytes, regardless of whether the diabetic nephropathy is type 1 or type 2.
What is the degradation target of podocyte autophagy? As mentioned previously, lysosomes are likely to be important in maintaining podocyte homeostasis. In our recent study, a massive accumulation of lysosomes with abnormal morphology was observed in the podocytes of obese type 2 diabetic rodents with autophagy deficiency [38]. Although it remains unclear whether lysosomes are a unique target of podocyte autophagy under any pathogenic conditions, our results suggest that damaged lysosomes are an important degradation target of podocyte autophagy, at least under diabetic conditions.
Compared with most other glomerular diseases, the renal outcomes of patients with diabetic nephropathy are extremely poor and the underlying mechanisms remain unclear. The severity of proteinuria-induced tubulointerstitial lesions is strongly correlated with renal outcomes regardless of the cause of the glomerular disease [14041]; therefore, diabetic conditions may exacerbate proteinuria-induced tubulointerstitial lesions compared with other primary glomerular diseases [42]. If so, identifying the detailed molecular mechanisms underlying the diabetes- and/or obesity-mediated vulnerability of proximal tubular cells may contribute to the development of new therapies that can improve renal outcomes in obese type 2 diabetes patients with persistent proteinuria. Is autophagy involved in the mechanism of diabetes-related vulnerability in proximal tubular cells? In this section, we discuss this possibility based on several recent publications.
The physiological role of autophagy in proximal tubular cells differs from its role in podocytes. Autophagy activity is very low in proximal tubular cells under basal conditions, but higher rates of autophagy are required by cells under stress conditions. Acute kidney injury due to nephrotoxic agents and ischemia is becoming a serious health problem in clinical settings. A great number of recent animal studies have shown that autophagy in proximal tubular cells is enhanced during acute kidney injury caused by ischemic-reperfusion and cisplatin, a nephrotoxic anti-cancer drug [434445]. Furthermore, mice lacking autophagy activity in proximal tubular cells, generated by deleting the Atg5 and Atg7 genes, showed progressive renal damage, suggesting that activation of autophagy during acute kidney injury is renoprotective [434446]. These mice also showed premature renal aging, suggesting that a low level of basal autophagy is essential to keep cell homeostasis in proximal tubular cells, or that autophagy induction is needed to cope with age-related extra- and intra-cellular stresses, such as hypoxia and ER stress.
Proteinuria creates strong nephrotoxic stress in a number of proteinuric kidney diseases, including diabetic nephropathy [140]. Based on our previous paper, an increased flux of protein into the urinary lumen from the glomeruli was found to activate autophagy in proximal tubular cells, which reabsorb the protein in urinary lumen. Atg5 knockout mice, deficient in proximal tubular cell-specific autophagy, developed severe proteinuria-induced tubulointerstitial lesions, along with enhanced proximal tubular cell apoptosis, similar to results obtained in animal models of acute kidney injury [47]. Collectively, proximal tubular cells can induce autophagy to cope with both acute and chronic nephrotoxic stresses. Thus, autophagy in proximal tubular cells and podocytes plays a renoprotective role in various stages of proteinuric kidney diseases.
Studies have also assessed the effects of obesity and diabetes on renoprotective autophagy in proximal tubular cells exposed to nephrotoxicity. Autophagy activity was shown to be significantly suppressed in the kidneys of streptozotocin-induced diabetic mice, HFD-induced obese mice and Wistar fatty rats [4748]. This led to the accumulation of damaged molecules and organelles, including p62 protein and damaged mitochondria, which are normally degraded via the autophagy-lysosomal pathway. Interestingly, autophagy insufficiency has been confirmed in renal biopsies of patients with obese type 2 diabetes [47]. The proximal tubular cells of patients with type 2 diabetes showed the accumulation of p62 protein, suggesting that deficient autophagy also occurs in humans with obesity type 2 diabetes.
The mechanism involved in autophagy-deficient proximal tubular cells of obese animals and humans has also been investigated. As mentioned in the previous section, autophagy is regulated by intracellular nutrient signals, such as mTORC1, AMPK, and Sirt1. Of these signals, mTORC1 is likely to be involved in diabetes-related inhibition of stress-induced autophagy in proximal tubular cells [47]. Histological analysis showed that the proximal tubular cells of obese type 2 diabetic mice and humans were intensely positive for phosphorylated S6 protein, an indicator of mTORC1 activation, and strongly associated with an obesity-related deficiency in autophagy [47]. These findings indicate that autophagy deficiency and the pathogenesis of diabetic nephropathy are closely associated. Obesity- or diabetes-mediated autophagy deficiency is likely to be involved in the vulnerability of proximal tubular cells (Fig. 3). Restoring autophagy activity may therefore be a new therapeutic strategy for diabetic patients with overt proteinuria.
Diabetes primarily injures endothelial cells, which is strongly associated with the initiation of diabetic nephropathy. Lenoir et al. [39] have shown direct evidence of the association between endothelial cell autophagy and diabetic nephropathy. They showed that endothelial cell-specific autophagy-deficient mice develop severe glomerular damage, indicating that autophagy in the endothelium also plays a renoprotective role against diabetic stress. Thus, although autophagy activity was not apparent in these cells from studying GFP-LC3 transgenic mice, cell-specific autophagy-deficient mice can provide im portant information regarding autophagy in diabetic nephropathy in renal cells other than podocytes and proximal tubular cells. This area is being actively researched and the role of diabetic autophagy in mesangial cells, distal tubular cells and collecting duct is under scrutiny.
The evolution of autophagy has enabled it to be activated under nutrient-depleted conditions, to overcome long-term periods of starvation. For the past several decades, many investigators have tried to identify calorie restriction-mediated anti-aging effects in mammals. Activation of autophagy is essential for calorie restriction-mediated life span elongation and anti-aging effects in various organisms [4950]. Autophagy in proximal tubular cells is activated by short-term starvation, suggesting that these cells possess a mechanism by which autophagy is induced in response to energy depletion. Calorie restriction has a renoprotective action against several kinds of renal injury [1951]. A calorie restriction regimen, which activates autophagy, should therefore become a potent therapeutic strategy to prevent diabetic nephropathy. Indeed, calorie restriction improves renal damage in type 2 diabetic Wistar fatty rats, as well as restoring autophagy activity in their proximal tubular cells [48]. Thus, an agent that can mimic caloric restriction may have potency to activate autophagy in mammalian cells, and become a therapy for diabetic nephropathy.
Given that autophagy is regulated by nutrient-responsive intracellular signals such as mTORC1, AMPK, and Sirt1, agents that can modify the activity of these signals may have therapeutic potency to treat diabetic nephropathy. Indeed, an mTORC1 inhibitor, rapamycin, improved glomerular lesions in experimental diabetic nephropathy, and it can activate autophagy; therefore, autophagy may be involved in the renoprotective mechanism of rapamycin in diabetic nephropathy. However, excessive mTORC1 inhibition also led to podocyte dysfunction [52]. Thus, whether mTORC1 inhibition is safe and effective for all patients with diabetic nephropathy is still under debate.
AMPK is a nutrient-sensing kinase that positively regulates autophagy. AMPK is activated under conditions of energy depletion and is likely suppressed in diabetic nephropathy [53]. Thus, AMPK-mediated induction of autophagy may be involved in its renoprotective mechanism. AMPK activation may be linked to autophagy for maintaining renal homeostasis in diabetic kidneys. AMPK is inactivated by dephosphorylation in the glomeruli and tubules of both type 1 and type 2 diabetic animal models. This inactivation is reversed by agents such as metformin and resveratrol, along with the attenuation of diabetic glomerular and tubular injury [5354555657]. Decreases in AMPK activity may be involved in the pathogenesis of diabetic nephropathy by reducing autophagy, suggesting that AMPK activation may be a target for restoring autophagy activity, even in diabetic kidneys. Some agents, such as 5-aminoimidazole-4-carboxamide ribonucleotide and metformin, can improve renal lesions in experimental diabetic nephropathy models. These agents might improve diabetic nephropathy through the restoration of autophagy activity in diabetic kidneys.
Sirt1 is another regulator of autophagy. A recent study has shown that Sirt1 deficiency is associated with the pathogenesis of both podocyte injury and proximal tubular cell damage in diabetic nephropathy, and that re-activation of this deacetylase improved diabetic nephropathy in mice [58]. We previously reported that an age-dependent decline of Sirt1 activity caused suppression of stress-induced autophagy in mouse kidneys, which accelerated the premature aging renal phenotype [19]. Thus, diabetes-related decline of Sirt1 activity may lead to inhibition of autophagy, leading to a severe renal phenotype in diabetic nephropathy. Taken together, these findings suggest that nutrient-sensing signals are strong candidate targets for modifying autophagy activity in kidney. However, direct evidence is still lacking and revealing the relationship among these signals, autophagy, and pathogenesis of diabetic nephropathy is a challenge that remains to be completed.
Over a period of decades, a large number of studies have attempted to reveal the molecular mechanisms underlying diabetic nephropathy and to develop new therapeutic strategies. However, the incidence of end-stage renal disease due to diabetic nephropathy continues to increase worldwide. There is an urgent need to identify new therapies for diabetic nephropathy. We have provided a perspective on the involvement of autophagy in the pathogenesis of diabetic nephropathy and whether it can be a new therapeutic target to treat this condition. The study of autophagy in diabetic nephropathy has only recently begun; therefore, evidence showing its involvement in the pathogenesis of diabetic nephropathy is still emerging. A great number of issues remain to be resolved. Future studies will provide clear evidence to determine whether autophagy should be considered a novel therapeutic target for diabetic nephropathy, and we hope that this review can contribute to the study of autophagy in diabetic nephropathy.
Figures and Tables
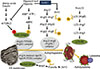 | Fig. 1Nutrient regulation of autophagy. Autohagosome formation is regulated by a number of autophagy-related proteins at multiple steps. Initiation of autophagy via Ulk1 complex is negatively and positively regulated by mammalian target of rapamycin complex 1 (mTORC1)- and 5'-AMP-activated protein kinase (AMPK)-dependent phosphorylation, respectively. Sirt1-dependent deacetylation is also involved in the activation of autophagy. Origin of autophagosome membrane is endoplasmic reticulum (ER) membrane. Autophagosome fuses with lysosome to form autolysosome and is finally degraded by lysosome enzymes. NAD, nicotinamide adenine dinucleotide; AMP, adenosine monophosphate; ATP, adenosine triphosphate; Atg, autophagy-related gene; LC3, light chain 3; Ulk1, unc-51-like kinase 1; PE, phosphatidylethanolamine; FOXO3a, forkhead box O3a. |
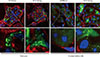 | Fig. 2Autophagy activity determined using green fluorescent protein light chain 3 (GFP-LC3) transgenic mouse. Autopphagome can be detected as GFP-LC3 dots in tissues of this mouse model. Autophagosomes formation is constitutively observed in podocytes even under ad-libitum condition. In contrast, autophagy can be observed in proximal tubular cells exposed to 48-hour fasting. The white dotted line box indicates the area for each enlarged figure. Blue signal, DAPI stain to visualize nuclei. Red signal, nidogen stain to visualize basement membrane. Green signal, GFP signal indicating LC3 protein. |
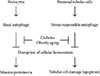 | Fig. 3Podocytes and proximal tubular cells have basal and stress-responsive autophagy, which is essential to maintain cellular homeostasis. Some pathological situations such as diabetes, obesity, and aging suppresses both basal and stress-responsive autophagy, leading to massive proteinuria and severe tubular cell damage. |
ACKNOWLEDGMENTS
This review manuscript supported by Grants-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science (No. 25713033 to SK, and No. 25670414 and No. 70242980 to DK); from the Takeda Science Foundation (to SK); from the Banyu Life Science Foundation International (to SK).
References
1. Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006; 17:2974–2984.
2. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005; 54:1615–1625.
3. Dunlop M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int Suppl. 2000; 77:S3–S12.
4. Forbes JM, Thallas V, Thomas MC, Founds HW, Burns WC, Jerums G, Cooper ME. The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. FASEB J. 2003; 17:1762–1764.
5. Ha H, Hwang IA, Park JH, Lee HB. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res Clin Pract. 2008; 82:Suppl 1. S42–S45.
6. Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998; 47:859–866.
7. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S;. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001; 345:861–869.
8. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993; 329:977–986.
9. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998; 352:837–853.
10. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995; 28:103–117.
11. Araki S, Haneda M, Sugimoto T, Isono M, Isshiki K, Kashiwagi A, Koya D. Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes. 2005; 54:2983–2987.
12. Yokoyama H, Araki S, Honjo J, Okizaki S, Yamada D, Shudo R, Shimizu H, Sone H, Moriya T, Haneda M. Association between remission of macroalbuminuria and preservation of renal function in patients with type 2 diabetes with overt proteinuria. Diabetes Care. 2013; 36:3227–3233.
13. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8:519–529.
14. Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010; 40:280–293.
15. Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009; 458:1131–1135.
16. Yoshizaki T, Kusunoki C, Kondo M, Yasuda M, Kume S, Morino K, Sekine O, Ugi S, Uzu T, Nishio Y, Kashiwagi A, Maegawa H. Autophagy regulates inflammation in adipocytes. Biochem Biophys Res Commun. 2012; 417:352–357.
17. Huber TB, Edelstein CL, Hartleben B, Inoki K, Jiang M, Koya D, Kume S, Lieberthal W, Pallet N, Quiroga A, Ravichandran K, Susztak K, Yoshida S, Dong Z. Emerging role of autophagy in kidney function, diseases and aging. Autophagy. 2012; 8:1009–1031.
18. Hartleben B, Godel M, Meyer-Schwesinger C, Liu S, Ulrich T, Kobler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstadt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest. 2010; 120:1084–1096.
19. Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A, Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010; 120:1043–1055.
20. Kume S, Thomas MC, Koya D. Nutrient sensing, autophagy, and diabetic nephropathy. Diabetes. 2012; 61:23–29.
21. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011; 147:728–741.
22. Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004; 432:1032–1036.
23. Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999; 402:672–676.
24. Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008; 90:313–323.
25. Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007; 131:1149–1163.
26. Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, Guan JL, Oshiro N, Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009; 20:1981–1991.
27. Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011; 13:1016–1023.
28. Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011; 13:132–141.
29. Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005; 6:298–305.
30. Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008; 105:3374–3379.
31. Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004; 15:1101–1111.
32. Kawachi H, Miyauchi N, Suzuki K, Han GD, Orikasa M, Shimizu F. Role of podocyte slit diaphragm as a filtration barrier. Nephrology (Carlton). 2006; 11:274–281.
33. Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997; 99:342–348.
34. Chen J, Chen MX, Fogo AB, Harris RC, Chen JK. mVps34 deletion in podocytes causes glomerulosclerosis by disrupting intracellular vesicle trafficking. J Am Soc Nephrol. 2013; 24:198–207.
35. Cina DP, Onay T, Paltoo A, Li C, Maezawa Y, De Arteaga J, Jurisicova A, Quaggin SE. Inhibition of MTOR disrupts autophagic flux in podocytes. J Am Soc Nephrol. 2012; 23:412–420.
36. Oshima Y, Kinouchi K, Ichihara A, Sakoda M, Kurauchi-Mito A, Bokuda K, Narita T, Kurosawa H, Sun-Wada GH, Wada Y, Yamada T, Takemoto M, Saleem MA, Quaggin SE, Itoh H. Prorenin receptor is essential for normal podocyte structure and function. J Am Soc Nephrol. 2011; 22:2203–2212.
37. Fang L, Zhou Y, Cao H, Wen P, Jiang L, He W, Dai C, Yang J. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS One. 2013; 8:e60546.
38. Tagawa A, Yasuda M, Kume S, Yamahara K, Nakazawa J, Chin-Kanasaki M, Araki H, Araki SI, Koya D, Asanuma K, Kim EH, Haneda M, Kajiwara N, Hayashi K, Ohashi H, Ugi S, Maegawa H, Uzu T. Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes. 2015; 09. 17. [Epub]. DOI: 10.2337/db15-0473.
39. Lenoir O, Jasiek M, Henique C, Guyonnet L, Hartleben B, Bork T, Chipont A, Flosseau K, Bensaada I, Schmitt A, Masse JM, Souyri M, Huber TB, Tharaux PL. Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy. 2015; 11:1130–1145.
40. Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992; 20:1–17.
41. Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968; 2:363–366.
42. Mallamaci F, Ruggenenti P, Perna A, Leonardis D, Tripepi R, Tripepi G, Remuzzi G, Zoccali C. ACE inhibition is renoprotective among obese patients with proteinuria. ACE inhibition is renoprotective among obese patients with proteinuria. J Am Soc Nephrol. 2011; 22:1122–1128.
43. Kimura T, Takabatake Y, Takahashi A, Kaimori JY, Matsui I, Namba T, Kitamura H, Niimura F, Matsusaka T, Soga T, Rakugi H, Isaka Y. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol. 2011; 22:902–913.
44. Liu S, Hartleben B, Kretz O, Wiech T, Igarashi P, Mizushima N, Walz G, Huber TB. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy. 2012; 8:826–837.
45. Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008; 294:F777–F787.
46. Takahashi A, Kimura T, Takabatake Y, Namba T, Kaimori J, Kitamura H, Matsui I, Niimura F, Matsusaka T, Fujita N, Yoshimori T, Isaka Y, Rakugi H. Autophagy guards against cisplatin-induced acute kidney injury. Am J Pathol. 2012; 180:517–525.
47. Yamahara K, Kume S, Koya D, Tanaka Y, Morita Y, Chin-Kanasaki M, Araki H, Isshiki K, Araki S, Haneda M, Matsusaka T, Kashiwagi A, Maegawa H, Uzu T. Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J Am Soc Nephrol. 2013; 24:1769–1781.
48. Kitada M, Takeda A, Nagai T, Ito H, Kanasaki K, Koya D. Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: a model of type 2 diabetes. Exp Diabetes Res. 2011; 2011:908185.
49. Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009; 325:201–204.
50. Fontana L, Partridge L, Longo VD. Extending healthy life span: from yeast to humans. Science. 2010; 328:321–326.
51. Cherry , Engelman RW, Wang BY, Kinjoh K, El-Badri NS, Good RA. Calorie restriction delays the crescentic glomerulonephritis of SCG/Kj mice. Proc Soc Exp Biol Med. 1998; 218:218–222.
52. Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Ruegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011; 121:2181–2196.
53. Kume S, Uzu T, Araki S, Sugimoto T, Isshiki K, Chin-Kanasaki M, Sakaguchi M, Kubota N, Terauchi Y, Kadowaki T, Haneda M, Kashiwagi A, Koya D. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J Am Soc Nephrol. 2007; 18:2715–2723.
54. Chang CC, Chang CY, Wu YT, Huang JP, Yen TH, Hung LM. Resveratrol retards progression of diabetic nephropathy through modulations of oxidative stress, proinflammatory cytokines, and AMP-activated protein kinase. J Biomed Sci. 2011; 18:47.
55. Takiyama Y, Harumi T, Watanabe J, Fujita Y, Honjo J, Shimizu N, Makino Y, Haneda M. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: a possible role of HIF-1alpha expression and oxygen metabolism. Diabetes. 2011; 60:981–992.
56. Ding DF, You N, Wu XM, Xu JR, Hu AP, Ye XL, Zhu Q, Jiang XQ, Miao H, Liu C, Lu YB. Resveratrol attenuates renal hypertrophy in early-stage diabetes by activating AMPK. Am J Nephrol. 2010; 31:363–374.
57. Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, Foretz M, Viollet B, Weinberg JM, Choudhury GG, Kasinath BS. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007; 292:F617–F627.
58. Hasegawa K, Wakino S, Simic P, Sakamaki Y, Minakuchi H, Fujimura K, Hosoya K, Komatsu M, Kaneko Y, Kanda T, Kubota E, Tokuyama H, Hayashi K, Guarente L, Itoh H. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med. 2013; 19:1496–1504.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download