Abstract
Background
Confirmation regarding the association between impaired fasting glucose (IFG) and biomarkers in addition to metabolic components and lifestyle factors are required in the occupational filed for preventing diabetes mellitus.
Methods
The study was performed in working men aged 30 to 60 years old, who were not taking medication for any metabolic diseases. The author measured the serum levels of high-sensitivity C-reactive protein (CRP), uric acid, and plasma fibrinogen as potential biomarkers of IFG.
Results
The mean serum uric acid, log-transformed serum CRP, and plasma fibrinogen levels were higher in the subjects with IFG than in those without IFG. Multivariate analysis revealed significant associations between the presence of IFG and age, log-transformed value of serum CRP, increased waist circumference, hypertension, and hypertriglyceridemia, with odds ratios of 1.1 (95% confidence interval [CI], 1.08 to 1.1; P<0.001), 1.8 (95% CI, 1.4 to 2.3; P<0.001), 1.3 (95% CI, 1.09 to 1.7; P<0.01), 1.9 (95% CI, 1.6 to 2.3; P<0.001), and 1.3 (95% CI, 1.04 to 1.6; P<0.05), respectively, for the presence of IFG.
Among the metabolic components, glucose intolerance is strongly related to lifestyles, including diet and exercise. In addition, other metabolic components such as central obesity, dyslipidemia and hypertension are mutually correlated to glucose intolerance in terms of the incidence or mortality of cardiovascular disease (CVD) [1].
Increased serum C-reactive protein (CRP) and plasma fibrinogen are important biomarker for the risk of CVD [2345]. In addition, meta-analysis of prospective studies has revealed hyperuricemia as a risk factor for type 2 diabetes mellitus (T2DM) [6], although the significance of hyperuricemia for predicting impaired fasting glucose (IFG) or T2DM was only noted in women [7].
The author recently reported that elevated serum level of CRP, uric acid, not habitual exercise and current smoking were associated with metabolic syndrome (MetS) [8], and there is a space of research with special emphasis on glucose intolerance as a key factor of metabolic components. Nakanishi et al. [9] reported clustering of components of MetS associated with diabetes precedes an increase in the risk of T2DM in Japanese men. They handled 3,298 male office workers, and more subclinical study in workers engaging in office and manufacturing job is needed to confirm the association. Namely, the association between metabolic components for IFG, biomarkers and lifestyle factors was evaluated as a subanalysis to understand the associations among the risk factors for CVD.
A total of 5,862 male workers of a car-manufacturing company in Japan, who attended an annual health examination in 2014, were enrolled in the study.
A self-administered questionnaire was used to record the history of tobacco smoking, alcohol intake, and habitual exercise. Smoking status was categorized as "never or past smoker" or "current smoker." As additional information, the author also used for categories of smoking such as "never smoker," "past smoker," "current smoker of under 20 cigarettes per day," or "current smoker of 20 or more cigarettes per day." Alcohol intake was classified by frequency as "everyday" or "sometimes or never." The author also defined "habitual exercise" as "walking or the same amount of exercise for more than 1 hour everyday." Patients receiving treatment for hypertension (n=403), dyslipidemia (n=227), diabetes mellitus (n=154), hyperuricemia (n=94), liver disease (n=8), cardiovascular and/or cerebrovascular disease (n=33) were excluded from this study. Furthermore, subjects with serum CRP ≥10.0 mg/L (n=82) were also excluded based on the possible presence of occult inflammatory or infectious disorder. After excluding the above subjects, the data of the remaining 5,102 male workers were analyzed in this study. Informed consent was obtained from each of the study participants, and IRB approval was made.
The National Cholesterol Education Program Adult Treatment Panel III (ATP III) criteria were used to define components of MetS such as central obesity (waist circumference ≥85 cm); hypertriglyceridemia (serum triglyceride ≥150 mg/dL [1.7 mmol/L]); low serum high density lipoprotein cholesterol (HDL-C; serum HDL <40 mg/dL [1.03 mmol/L]); high-blood pressure (systolic blood pressure ≥130 mm Hg and/or a diastolic blood pressure ≥85 mm Hg); high fasting glucose (≥100 mg/dL [5.6 mmol/L]) [10]. The cutoff point for plasma fasting glucose was modified according to the criteria of International Diabetes Federation [11].
After each subject had rested for 5 minutes, the brachial SBP and DBP were measured twice using an automated blood pressure measurement device (TM-2540C; A & D Co., Ltd., Tokyo, Japan), and average value of the seated blood pressure measurements was used for the analysis. The waist circumference at the level of the umbilicus was measured at the end of expiration.
Fasting blood samples were obtained from each subject with no intake of food for 12 hours. The serum triglyceride and HDL-C concentrations were measured enzymatically. The concentrations of plasma glucose were determined using the glucose oxidase-oxygen electrode method.
Serum uric acid was measured with an automatic analyzer (7700 series; Hitachi, Tokyo, Japan). Serum high-sensitivity CRP was measured by a latex turbidity assay (Mitsubishi Kagaku Iatron, Tokyo, Japan) using the Hitachi 7700 auto-analyzer. The lower detection limit of this assay was 0.1 mg/L. The intra-assay coefficient of variation (CV) for repeated measurements ranged from 0.84% to 2.54%. Plasma fibrinogen was measured using CA-1500 (Sysmex Inc., Kobe, Japan) by the light scattering method. The lower detection limit of this assay was 250 mg/L. The intra-assay CV for repeated measurements ranged from 3.26% to 6.99%. Serum HDL-C, triglyceride, and glucose levels were determined enzymatically with a Hitachi 7700 auto-analyzer.
Serum CRP was log-transformed, and the log mean value was transformed back and presented as geometric means.
Differences in the mean value or frequency between the two groups stratified by the presence of IFG were checked for significance by the Mann-Whitney test or Fisher exact test. The Jonckheere-Terpstra trend test was applied against the four categories of smoking. Then, the log-transformed values of serum CRP, serum uric acid, plasma fibrinogen, age, and the histories of smoking, drinking and regular exercise, and four components of MetS were used as independent variables in a logistic regression analysis carried out to determine their associations with IFG. SPSS version 21.0 (IBM Co., Armonk, NY, USA) was used for the analysis. Statistical significance was set at three levels such as P<0.05, P<0.01, and P<0.001, respectively.
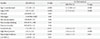
The subjects ranged in age from 30 to 60 years, with a mean age ± standard deviation of 42.6±6.9 years. The basic characteristics of the subjects, including the lifestyle factors, are listed in Table 1. Prevalence of IFG was 11.2% (573/5,102). Statistically significant differences for all the variables were observed between the two groups stratified by the presence/absence of IFG, except for smoking.
Trend analysis for the mean values of the three biological factors was conducted according to the smoking category (Table 2). After adjustment for age, the values of all the factors increased or decreased significantly with changes of the smoking category.
Multiple logistic regression analysis was conducted to identify the factors significantly associated with the IFG (Table 3). The odds ratios (95% confidence intervals [CIs]) of advanced age, elevated log-transformed value of serum CRP, increased waist circumference, hypertension, and hypertriglyceridemia for the presence of IFG were 1.1 (95% CI, 1.08 to 1.1; P<0.001), 1.8 (95% CI, 1.4 to 2.3; P<0.001), 1.3 (95% CI, 1.09 to 1.7; P<0.01), 1.9 (95% CI, 1.6 to 2.3; P<0.001), and 1.3 (95% CI, 1.04 to 1.6; P<0.05), respectively. In contrast, there was no significant association between the IFG and lifestyle factors, plasma fibrinogen or serum uric acid. Stepwise multiple logistic regression analysis (Wald method) was also applied, which showed the same results (Table 3).
In this study, serum levels of uric acid and plasma fibrinogen were not identified as being significantly associated with IFG. In contrast, advanced age, elevated plasma CRP and three metabolic components, namely, increased waist circumference, hypertension, and hypertriglyceridemia were significantly associated with the IFG.
Raynaud et al. [12] reported the existence of a significant negative correlation between plasma fibrinogen and insulin sensitivity, and existence of a positive correlation between plasma fibrinogen and basal insulin after adjustment for the body mass index, body fat mass, and waist-to-hip ratio, which had been reported by the same authors [13]. Although plasma fibrinogen was not selected as a surrogate biological marker for IFG in our study, a prospective study is warranted to clarify the effect of plasma fibrinogen on the incident IFG.
Simental-Mendia et al. [14] reported an association between elevated serum CRP and IFG in obese subjects, and the same result was observed in our study after adjustment for the waist circumference. Ye et al. [15] also reported a significant association between elevated serum CRP and increased glycosylated hemoglobin in both genders within the euglycemia range after adjustments for multiple factors. More prospective studies are needed to confirm the predictive ability of the serum CRP for IFG.
Three lifestyle factors were identified as having no predictive ability for IFG. The mean values of plasma fibrinogen, serum CRP, and serum uric acid changed according to the smoking category, and only serum CRP was selected as a significant biomarker of IFG in our study. Fagard and Nilsson [16] sounded a caution against smoking for diabetes, and I also found a high prevalence of smoking in patients with diabetes mellitus [1718]. The author conducted a cross-sectional study to clarify the risk factors for IFG, and causality of the association cannot be confirmed. This association should be checked by a follow-up study.
This report has an advantage of presenting risk factors for IFG in male workers in Japan, with special emphasis on systemic inflammation and other metabolic components.
Figures and Tables
Table 1
Characteristics of the subjects categorized according to the presence/absence of impaired fasting glucose

Values are presented as arithmetic mean±standard deviation or percentage (number). Data for triglycerides and CRP were presented as geometric mean (geometric standard deviation). Mann-Whitney test for continuous variables or Fisher exact test for categorical variables.
IFG, impaired fasting glucose; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high density lipoprotein cholesterol; CRP, C-reactive protein.
aNumbers of IFG and non-IFG were 569 and 4,515, respectively.
Table 2
Characteristics of the subjects categorized according to the smoking status

Table 3
Multiple logistic regression analysis to identify the variables showing independent associations with impaired fasting glucose
ACKNOWLEDGMENTS
I wish to express my appreciation to the study participants. This study was supported by Grant-in-Aid for Scientific Research (C) (24590763).
References
1. Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012; 59:635–643.
2. Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010; 74:213–220.
3. Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010; 375:132–140.
4. Fibrinogen Studies Collaboration. Kaptoge S, White IR, Thompson SG, Wood AM, Lewington S, Lowe GD, Danesh J. Associations of plasma fibrinogen levels with established cardiovascular disease risk factors, inflammatory markers, and other characteristics: individual participant meta-analysis of 154,211 adults in 31 prospective studies: the fibrinogen studies collaboration. Am J Epidemiol. 2007; 166:867–879.
5. Ahmadi-Abhari S, Luben RN, Wareham NJ, Khaw KT. Seventeen year risk of all-cause and cause-specific mortality associated with C-reactive protein, fibrinogen and leukocyte count in men and women: the EPIC-Norfolk study. Eur J Epidemiol. 2013; 28:541–550.
6. Lv Q, Meng XF, He FF, Chen S, Su H, Xiong J, Gao P, Tian XJ, Liu JS, Zhu ZH, Huang K, Zhang C. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One. 2013; 8:e56864.
7. Yamada T, Fukatsu M, Suzuki S, Wada T, Joh T. Elevated serum uric acid predicts impaired fasting glucose and type 2 diabetes only among Japanese women undergoing health check-ups. Diabetes Metab. 2011; 37:252–258.
8. Kawada T, Otsuka T, Inagaki H, Wakayama Y, Katsumata M. Biological markers, lifestyles and metabolic syndrome in workers. Diabetes Metab Syndr. 2015; 9:71–73.
9. Nakanishi N, Nishina K, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Clustering of components of the metabolic syndrome and risk for development of type 2 diabetes in Japanese male office workers. Diabetes Res Clin Pract. 2004; 63:185–194.
10. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106:3143–3421.
11. Alberti KG, Zimmet P, Shaw J. IDF Epidemiology Task Force Consensus Group. The metabolic syndrome: a new worldwide definition. Lancet. 2005; 366:1059–1062.
12. Raynaud E, Perez-Martin A, Brun J, Aissa-Benhaddad A, Fedou C, Mercier J. Relationships between fibrinogen and insulin resistance. Atherosclerosis. 2000; 150:365–370.
13. Raynaud E, Brun JF, Perez-Martin A, Orsetti A, Solere M. Negative correlation between plasma fibrinogen and insulin sensitivity measured with the minimal model technique. Clin Hemorheol Microcirc. 1998; 18:323–330.
14. Simental-Mendia LE, Lazalde B, Zambrano-Galvan G, Simental-Saucedo L, Rabago-Sanchez E, Rodriguez-Moran M, Guerrero-Romero F. Relation between C-reactive protein and impaired fasting glucose in obese subjects. Inflammation. 2012; 35:1742–1746.
15. Ye X, Franco OH, Yu Z, Li H, Hu FB, Liu H, Wang X, Tang H, Liu Y, Chen Y, Lin X. Associations of inflammatory factors with glycaemic status among middle-aged and older Chinese people. Clin Endocrinol (Oxf). 2009; 70:854–862.
16. Fagard RH, Nilsson PM. Smoking and diabetes: the double health hazard! Prim Care Diabetes. 2009; 3:205–209.
17. Kawada T. Difficulties encountered by diabetic workers in a company trying to stop smoking. J Diabetes. 2011; 3:317.
18. Kawada T. The association between physical data, mental status and blood rheology with special emphasis on smoking status, depressive state, and blood viscosity. Circ J. 2011; 75:1282.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download