See the reply "Response: GDF15 Is a Novel Biomarker for Impaired Fasting Glucose (Diabetes Metab J 2014;38:472-9)" in Volume 39 on page 84.
Abstract
Background
Growth differentiation factor-15 (GDF15) is a protein that belongs to the transforming growth factor β superfamily. An elevated serum level of GDF15 was found to be associated with type 2 diabetes mellitus (T2DM). T2DM is an inflammatory disease that progresses from normal glucose tolerance (NGT) to impaired fasting glucose (IFG). Hence, we aimed to validate the relationship between GDF15 and IFG.
Methods
The participants were divided into the following three groups: NGT (n=137), IFG (n=29), and T2DM (n=75). The controls and T2DM outpatients visited the hospital for routine health check-ups. We used fasting blood glucose to detect IFG in nondiabetic patients. We checked the body mass index (BMI), C-reactive protein level, metabolic parameters, and fasting serum GDF15 level.
Results
Age, BMI, triglyceride, insulin, glucose, homeostatic model assessment-insulin resistance (HOMA-IR), and GDF15 levels were elevated in the IFG and T2DM groups compared to the NGT group. In the correlation analysis between metabolic parameters and GDF15, age and HOMA-IR had a significant positive correlation with GDF15 levels. GDF15 significantly discriminated between IFG and NGT, independent of age, BMI, and HOMA-IR. The serum levels of GDF15 were more elevated in men than in women. As a biomarker for IFG based on the receiver operating characteristic curve analysis, the cutoff value of GDF15 was 510 pg/mL in males and 400 pg/mL in females.
According to the 2011 National Diabetes Fact Sheet of the American Diabetes Association (ADA), 25.6 million people (11.3%) in the United States aged 20 years or older had diabetes in 2010, and 35% of United States adults had prediabetes (50% of adults aged 65 years or older) [1]. In Korea, approximately 4 million Korean people (12.4%) aged 30 years or older had diabetes in 2011, and 20% of Korean adults had prediabetes impaired fasting glucose (IFG) [2]. Recently, there has been a striking increase in the prevalence of type 2 diabetes mellitus (T2DM) as well as that of prediabetes. In the prediabetic stage, lifestyle modifications and the use of some drugs such as metformin and α-glucosidase inhibitor can modify insulin resistance (IR) and hence delay or reduce the progression to T2DM [3,4,5,6]. To achieve this outcome, it is necessary to identify prediabetic patients earlier with the use of a biomarker. Several markers for predicting prediabetes have been proposed. Acute phase proteins (C-reactive protein [CRP], plasminogen activator inhibitor-1) and coagulation factors (fibrinogen, D-dimer) are considered markers that can predict prediabetes [7,8]. However, these parameters are more correlated with cardiovascular risk than IR. Therefore, a novel marker that is based on the pathogenesis of prediabetes and T2DM is necessary.
IR is already present in the prediabetic stage. T2DM develops when the IR becomes more severe and the pancreatic β-cells fail to compensate for IR [9]. The sequential progression from normal glucose tolerance (NGT) to T2DM through prediabetes is associated with multifactorial components. Chronic inflammation may be a contributing factor for the development of T2DM in a nondiabetic population. In a large cohort study, patients with prediabetes who had high levels of acute phase proteins (e.g., CRP, plasminogen activator inhibitor-1) converted to T2DM more frequently than those who had lower levels of acute phase proteins [7,10]. Because T2DM is an inflammatory disease, treatment with salicylates and interleukin-1 (IL-1) antagonists lowered blood glucose levels and attenuated the T2DM-associated complications [11]. Inflammation was associated with increased IR rather than decreased insulin secretion. Inflammation in the prediabetic stage accentuated the cardiovascular risk by more than 1.56 times that in the NGT group [12,13,14].
Growth differentiation factor-15 (GDF15) is a divergent member of the transforming growth factor-β (TGF-β) superfamily [15]. The role and the downstream mechanism of GDF15 have not yet been clearly elucidated. According to many studies, GDF15 is associated with cancers, cardiovascular diseases, and inflammatory diseases. The putative role of GDF15 is that of a stress- or inflammation-responsive cytokine. As one of the aspects of the inflammatory disease of T2DM, elevated levels of GDF15 were found to be associated with the presence of T2DM and the future development of T2DM [16,17,18]. However, the relationship between GDF15 and prediabetes has not yet been investigated.
In the prediabetic stage, patients with IFG had less severe IR and lower cardiovascular risk than patients with impaired glucose tolerance (IGT) [19]. Therefore, we aimed to evaluate GDF15 as a biomarker for discriminating patients with IFG from a nondiabetic population.
We recruited 241 participants from the Chungnam National University Hospital from June 2012 to May 2013. The participants were divided into three groups: NGT, IFG, and T2DM. The patients with T2DM were outpatients of the Department of Endocrinology. The classification of hyperglycemia was based on ADA criteria, with IFG defined as a fasting plasma glucose level from 100 to 125 mg/dL [20]. In the routine health check-up population, five patients were found to be newly diagnosed with T2DM and were included in the T2DM group. We excluded five patients with a known malignancy (three cases of thyroid cancers, one case of stomach cancer, and one case of breast cancer). We also excluded three patients with an elevated aspartate transaminase (AST)/alanine transaminase (ALT) (above 100 IU/L). We then compared the clinical characteristics, biochemical data, and serum GDF15 levels among the three groups.
We collected blood samples using ethylenediaminetetraacetic acid tubes in the morning after an overnight fast of more than 8 hours and checked the fasting blood glucose level, lipid profile (high density lipoprotein cholesterol [HDL-C], low density lipoprotein cholesterol [LDL-C], total cholesterol, triglycerides) using a blood chemistry analyzer (Hitachi 747; Hitachi, Tokyo, Japan). Insulin was quantified using an immunoradiometric assay kit (DIAsource INS-IRMA Kit; DIAsource, Louvain-la-Neuve, Belgium). Glycosylated hemoglobin was measured using high-performance liquid chromatography (BioRad, Hercules, CA, USA). AST and ALT activities were measured by the International Federation of Clinical Chemistry Ultra Violet method without pyridoxal phosphate (TBA-2000FR; Toshiba, Tokyo, Japan). CRP was measured by the photometric latex agglutination method (TBA-2000FR). Homeostatic model assessment-IR (HOMA-IR) was calculated as fasting serum insulin (µU/mL)×fasting plasma glucose (mmol/L)/22.5, and HOMA-β was calculated as fasting plasma insulin (µU/mL)×20/fasting plasma glucose (mmol/L)-3.5. The fasting serum GDF15 level was measured by quantitative sandwich enzyme immunoassay technique using enzyme-linked immunosorbent assay (ELISA; R&D systems, Minneapolis, MN, USA; Quantikine ELISA, Human GDF15, catalog number: DGD150).
All of the parameter values were calculated as the mean±standard deviation. Statistical significance was defined as P<0.05 (two-tailed analysis). One-way analysis of variance was used to compare the clinical characteristics, biochemical data, and GDF15 levels among the three groups. To analyze the strength of the relationship between GDF15 and the studied variables, pearson correlation coefficients were used. Logistic regression analysis was performed to evaluate the contribution of GDF15 in predicting IFG in nondiabetic participants. For determining the predictive value of GDF15 for IFG, we quantified GDF15 by constructing receiver operating characteristic curves and measured the area under the curve (AUC). Statistical analysis was performed using SPSS version 20 (IBM Co., Armonk, NY, USA).
The clinical characteristics of the study population are shown in Table 1. The patients in the T2DM group (55.41±12.8 years) were significantly older than the patients in the IFG (50.14±9.86 years) and NGT (45.48±9.50 years) groups. Body mass index (BMI) was the highest in the T2DM group (26.42±4.9 kg/m2), while BMI in the IFG group (25.44±2.80 kg/m2) was significantly higher than that in the NGT group (24.34±3.10 kg/m2). With respect to the lipid profile, total cholesterol, and LDL levels were lower in the T2DM group than in the IFG and NGT groups. Fasting serum glucose and insulin levels increased stage by stage from NGT to T2DM through IFG. The calculated HOMA-IR level was elevated in the IFG group (2.51±1.60), and it was the highest in the T2DM group (4.37±4.20). HOMA-IR levels in the T2DM group and IFG group showed the presence of IR.
The acute phase protein CRP, which is a marker of inflammation, was elevated only in the T2DM group (1.32±2.0 mg/L). There was no difference in the CRP level (P=1.00) between the NGT group (0.51±0.8 mg/L) and the IFG group (0.30±0.3 mg/L). The calculated HOMA-β, which measures the insulin secretion function of pancreatic β-cells, significantly declined from the NGT to T2DM groups through the IFG group.
The fasting serum GDF15 level was the lowest in the NGT group (484.05±291.0 pg/mL) and the highest in the T2DM group (866.64±628.10 pg/mL). The IFG group showed an intermediate value of fasting serum GDF15 (700.79±501.20 pg/mL), and all of the GDF15 values were significantly different among the three groups (Fig. 1).
By Pearson coefficient correlation analysis, GDF15 was strongly associated with age (r=0.367, P<0.001), glucose (r=0.249, P<0.001), and HOMA-IR (r=0.311, P<0.001) (Table 2). GDF15 was negatively correlated with total cholesterol (r=-0.224, P=0.001), LDL-C (r=-0.200, P=0.004), and HDL-C (r=-0.175, P=0.011). There was no significant correlation between GDF15 and CRP (r=0.040, P=0.616).
In logistic regression analysis, HOMA-IR significantly contributed to our ability to discriminate IFG from NGT (odds ratio [OR], 2.114; P=0.014). GDF15 also significantly discriminated IFG from NGT (OR, 1.532; P=0.005) after adjusting for age and BMI (Table 3).
We evaluated GDF15 as a marker for discriminating patients with IFG from nondiabetic patients after the exclusion of known T2DM patients. The mean values of GDF15 differed between men and women. Fasting serum GDF15 levels were higher in men than in women (717.61±520.20 and 503.99±396.10 pg/mL, respectively; P=0.002). The total AUC of GDF15 was 0.682, sensitivity was 69.1%, specificity was 67.5%, and the cutoff value was 470 pg/mL. In men, the AUC of GDF15 was 0.679, sensitivity was 68.6%, specificity was 66.8%, and the cutoff value was 510 pg/mL. In women, the AUC of GDF15 was 0.698, sensitivity was 67.6%, specificity was 68.0%, and the cutoff value was 400 pg/mL (Fig. 2).
Prediabetes is usually unrecognized and therefore constitutes a major public health concern that needs earlier intervention because the majority of cases in the prediabetic stage proceed to T2DM [21]. The 75 g oral glucose tolerance test (OGTT) is the gold standard to identify prediabetic patients, including patients with IFG and IGT [22]. However, it takes more than 2 hours to perform the 75 g OGTT, and the consumption of 75 g of glucose occasionally causes gastrointestinal symptoms; hence, there is a limitation to performing the 75 g OGTT as a screening test for detecting prediabetes. The other representative markers for identifying prediabetes are fasting blood glucose and hemoglobin A1c (HbA1c). However, these parameters have usage limitations. Specifically, the fasting blood glucose level is affected by many factors. Diurnal variation, hormonal influence, consumption of drugs and medications, alcohol intake, and accompanying disease state may be associated with the variation of blood glucose levels [23]. Other than the stage of prediabetes or diabetes, a recent meal may also affect the fasting blood glucose level. In the animal experiments performed by Raher et al. [24], a short-duration intervention of a high-fat diet (9 days) provoked IR and elevated the fasting glucose level in C57BL/6 mice. HbA1c is also affected by many factors; however, it is convenient to measure this parameter as the patients do not need to fast, thereby enabling random blood sampling and making it a more comprehensive measure of mean glycemia than fasting plasma glucose [25]. Factors that influence HbA1c are anemia, hemoglobinopathies, liver disease, and the ingestion of alcohol, vitamin C and vitamin E, etc. Furthermore, the differences between days, between subjects and the standardization of HbA1c measurement assays lead to difficulty in maintaining consistency [26]. In spite of the lack of consistency, fasting blood glucose and HbA1c are effective for predicting T2DM [27]. However, as markers for identifying prediabetes, HbA1c and fasting blood glucose were found to be less sensitive than plasma glucose at 2-hour after glucose loading [28]. Fasting blood glucose and HbA1c are glucose-dependent biochemical data that are continuously changing due to many factors. Therefore, it is necessary to identify a biomarker to detect prediabetes by a method that is not based on plasma glucose to maintain the consistency and ease of preparation. GDF15 is a nonglucose protein, and only one fasting blood sample is needed for its measurement. Furthermore, serum GDF15 levels were found to be stable at room temperature for 48 hours and showed a consistent value despite four freeze/thaw cycles (20 hours at -70℃ followed by 4 hours at room temperature). In addition to these advantages, the anticoagulant used for sampling does not affect the measurements [29]. However, further prospective studies on GDF15 measurement are required for analyzing the effects of meals, accompanying diseases, and drugs.
GDF15 is a divergent member of the TGF-β superfamily. It was initially named macrophage inhibitory cytokine-1 (MIC-1) [15] and is sometimes also known as placental bone morphogenetic protein (PLAB) [30], placental transforming growth factor-β (PTGF-β) [31], prostate derived factor (PDF) [32], and nonsteroidal anti-inflammatory drug-activated protein-1 (NAG-1) [33]. Initially, GDF15 was regarded as an anti-inflammatory protein that inhibits macrophage activation; therefore, the induction of GDF15 reduces tumor necrosis factor-α (TNF-α) secretion in macrophages [15]. The diverse roles of GDF15 were further elucidated in additional studies; however, the exact mechanism and downstream signal are not known. The GDF15 levels were elevated more in T2DM patients than in the non-T2DM population. Insulin resistance is induced by macrophage infiltration and inflammation in the liver, adipose tissues, and muscles. Elevated levels of proinflammatory cytokines, for example, TNF-α and IL-6, have been found in adipose tissues and blood samples from IR patients with diabetes [34]. Weight reduction to reduce TNF-α in obese and insulin-resistant patients improved the IR in an animal model [16]. GDF15 was also identified in adipose tissues where it is secreted as an adipokine [17].
Therefore, GDF15 may be a biomarker for predicting IR and diabetes. In the Xenical in the Prevention of Diabetes in Obese Subjects (XENDOS) trial, obese people with high levels of GDF15 progressed to T2DM during the 4-year follow-up period [18]. Dostalova et al. [35] also reported that patients with obesity and T2DM showed increased serum concentrations of GDF15. In a clinical experiment, Karczewska-Kupczewska et al. [36] reported that there was an inverse correlation between insulin sensitivity and GDF15 values during the clamp examination. However, the relationship between GDF15 and prediabetes has not yet been investigated. Prediabetes can also develop due to IR, and it is associated with an increased risk of diabetes and cardiovascular diseases in the future [10]. In the XENDOS trial, the IFG group showed no significant change compared with the NGT group. In addition, the mean GDF15 level in the NGT group was 869 pg/mL and that in the T2DM group was 1,136 pg/mL because all of the participants were obese patients with a mean BMI of 37.6 kg/m2. In a study by Dostalova et al. [35], the GDF15 level in the NGT group was 330 pg/mL and that in the T2DM group was 1,100 pg/mL. In the NGT group, the mean GDF15 level in the nondiabetic obese people with a mean BMI of 43.2 kg/m2 was approximately 530 pg/mL. The GDF15 levels of the aforementioned two studies were more elevated than in our study, possibly because the high BMI of participants might affect the values. In our study, the total mean GDF15 level in the NGT group was 484.05 pg/mL (445.02 pg/mL in men and 378.68 pg/mL in women). These data are similar to those in nonobese people in the NGT group (GDF15, 380 pg/mL) in the study by Dostalova et al. [35]. The total GDF15 level in the T2DM group was 866.64 pg/mL (919.0 pg/mL in men and 722.88 pg/mL in women). Our values in the T2DM group were slightly lower than those in the other studies because the BMI of our participants was 24 to 26 kg/m2. According to previous data, GDF15 levels are correlated with BMI and T2DM. In our study, although BMI was significantly different among the NGT, IFG, and T2DM groups (P<0.001), most of the participants' BMIs were below 30 kg/m2. When comparing the GDF15 levels by BMI, there was no significant difference between groups (data not shown). Therefore, we could postulate the relationship between GDF15 and IR without the effect of obesity and discriminate IFG from NGT using the GDF15 measurement.
In our study, GDF15 showed a significant correlation with age and also showed a difference by sex. Similar patterns were observed in the XENDOS trial. Because aging is also a chronic inflammatory process, older patients showed higher levels of GDF15 [37]. GDF15 levels were more elevated in males than in females (717.61±520.20, 503.99±396.10 pg/mL, P=0.002). However, the reference values according to the age group and sex have not been elucidated, and therefore, a large-scale study is needed. In addition, GDF15 showed a significant correlation with lipid profiles. Total cholesterol and LDL-C were lower in the T2DM group. We did not check whether any of the participants were take lipid-lowering agents, which might affect the correlation with GDF15.
To validate the role of GDF15 as a predictor of IFG, a prospective study is required. However, it is a limitation that our study was a cross-sectional study that only discriminated IFG from NGT. Furthermore, we did not perform the 75 g OGTT in all of the participants. The patients in the IFG group combined with the IGT group might be included in the IFG group in our study, and the patients in the NGT group with hidden IGT or hidden T2DM might be included. It is necessary to plan a study to identify the exact role of GDF15 in prediabetes with isolated IFG, isolated IGT, and combined IFG with IGT using 75 g OGTT and HbA1c. In conclusion, it is convenient to measure the GDF15 level with one fasting sample, and consistent protein stability data were observed. The GDF15 level is based on the response to chronic inflammation and its compensatory secretion in IFG and T2DM. GDF15 is highly associated with IR, and the levels were significantly different between the NGT and IFG groups. Hence, GDF15 might be a novel biomarker for detecting IFG.
Figures and Tables
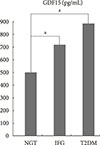 | Fig. 1The differences in growth differentiation factor-15 (GDF15) levels among the normal glucose tolerance (NGT), impaired fasting glucose (IFG), and type 2 diabetes mellitus (T2DM) groups. aP<0.001. |
 | Fig. 2Prediction of impaired fasting glucose (IFG) based on the receiver operating characteristic (ROC) curve of growth differentiation factor-15 (A, total; B, male; C, female). |
Table 1
Clinical characteristics and comparison between metabolic parameters and GDF15 among the NGT, IFG, and T2DM groups

Values are presented as mean±standard deviation.
GDF15, growth differentiation factor-15; NGT, normal glucose tolerance; IFG, impaired fasting glucose; T2DM, type 2 diabetes mellitus; BMI, body mass index; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; TG, triglyceride; WBC, white blood cell; CRP, C-reactive protein; HOMA-IR, homeostatic model assessment-insulin resistance; HOMA-β, homeostatic model assessment β-cell function; AST, aspartate transaminase; ALT, alanine transaminase.
ACKNOWLEDGMENTS
This study was supported by financial support from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A2A1A03002833), MOE, and the Korean Healthcare Technology R&D project (A100588), MHW, Korea.
References
1. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention;2011.
2. Korea Centers for Disease Control and Prevention (KCDC) and the Korean Ministry of Health and Welfare: Diabetes fact sheet in Korea 2012. updated 2014 Oct 8. Available from: http://www.diabetes.or.kr/temp/diabetes_factsheet_2013111.pdf.
3. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346:393–403.
4. Hanefeld M. Cardiovascular benefits and safety profile of acarbose therapy in prediabetes and established type 2 diabetes. Cardiovasc Diabetol. 2007; 6:20.
5. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001; 344:1343–1350.
6. Goldberg R, Temprosa M, Otvos J, Brunzell J, Marcovina S, Mather K, Arakaki R, Watson K, Horton E, Barrett-Connor E. Lifestyle and metformin treatment favorably influence lipoprotein subfraction distribution in the Diabetes Prevention Program. J Clin Endocrinol Metab. 2013; 98:3989–3998.
7. Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999; 353:1649–1652.
8. Coban E, Sari R, Ozdogan M, Akcit F. Levels of plasma fibrinogen and d-dimer in patients with impaired fasting glucose. Exp Clin Endocrinol Diabetes. 2005; 113:35–37.
9. Kahn BB. Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell. 1998; 92:593–596.
10. Festa A, D'Agostino R Jr, Tracy RP, Haffner SM. Insulin Resistance Atherosclerosis Study. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002; 51:1131–1137.
11. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011; 11:98–107.
12. Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999; 22:233–240.
13. Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004; 164:2147–2155.
14. Deedwania PC, Fonseca VA. Diabetes, prediabetes, and cardiovascular risk: shifting the paradigm. Am J Med. 2005; 118:939–947.
15. Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997; 94:11514–11519.
16. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995; 95:2409–2415.
17. Ding Q, Mracek T, Gonzalez-Muniesa P, Kos K, Wilding J, Trayhurn P, Bing C. Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology. 2009; 150:1688–1696.
18. Kempf T, Guba-Quint A, Torgerson J, Magnone MC, Haefliger C, Bobadilla M, Wollert KC. Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: results from the XENDOS trial. Eur J Endocrinol. 2012; 167:671–678.
19. Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999; 22:920–924.
20. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014; 37:Suppl 1. S81–S90.
21. Bergman M. Inadequacies of absolute threshold levels for diagnosing prediabetes. Diabetes Metab Res Rev. 2010; 26:3–6.
22. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998; 15:539–553.
23. Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability: does it matter? Endocr Rev. 2010; 31:171–182.
24. Raher MJ, Thibault HB, Buys ES, Kuruppu D, Shimizu N, Brownell AL, Blake SL, Rieusset J, Kaneki M, Derumeaux G, Picard MH, Bloch KD, Scherrer-Crosbie M. A short duration of high-fat diet induces insulin resistance and predisposes to adverse left ventricular remodeling after pressure overload. Am J Physiol Heart Circ Physiol. 2008; 295:H2495–H2502.
25. Rohlfing C, Wiedmeyer HM, Little R, Grotz VL, Tennill A, England J, Madsen R, Goldstein D. Biological variation of glycohemoglobin. Clin Chem. 2002; 48:1116–1118.
26. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009; 1:9–17.
27. Sato KK, Hayashi T, Harita N, Yoneda T, Nakamura Y, Endo G, Kambe H. Combined measurement of fasting plasma glucose and A1C is effective for the prediction of type 2 diabetes: the Kansai Healthcare Study. Diabetes Care. 2009; 32:644–646.
28. Lorenzo C, Wagenknecht LE, Hanley AJ, Rewers MJ, Karter AJ, Haffner SM. A1C between 5.7 and 6.4% as a marker for identifying pre-diabetes, insulin sensitivity and secretion, and cardiovascular risk factors: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care. 2010; 33:2104–2109.
29. Kempf T, Horn-Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, Drexler H, Johnston N, Wallentin L, Wollert KC. Circulating concentrations of growth-differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem. 2007; 53:284–291.
30. Hromas R, Hufford M, Sutton J, Xu D, Li Y, Lu L. PLAB, a novel placental bone morphogenetic protein. Biochim Biophys Acta. 1997; 1354:40–44.
31. Lawton LN, Bonaldo MF, Jelenc PC, Qiu L, Baumes SA, Marcelino RA, de Jesus GM, Wellington S, Knowles JA, Warburton D, Brown S, Soares MB. Identification of a novel member of the TGF-beta superfamily highly expressed in human placenta. Gene. 1997; 203:17–26.
32. Paralkar VM, Vail AL, Grasser WA, Brown TA, Xu H, Vukicevic S, Ke HZ, Qi H, Owen TA, Thompson DD. Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family. J Biol Chem. 1998; 273:13760–13767.
33. Baek SJ, Kim JS, Moore SM, Lee SH, Martinez J, Eling TE. Cyclooxygenase inhibitors induce the expression of the tumor suppressor gene EGR-1, which results in the up-regulation of NAG-1, an antitumorigenic protein. Mol Pharmacol. 2005; 67:356–364.
34. Tajiri Y, Mimura K, Umeda F. High-sensitivity C-reactive protein in Japanese patients with type 2 diabetes. Obes Res. 2005; 13:1810–1816.
35. Dostalova I, Roubicek T, Bartlova M, Mraz M, Lacinova Z, Haluzikova D, Kavalkova P, Matoulek M, Kasalicky M, Haluzik M. Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. Eur J Endocrinol. 2009; 161:397–404.
36. Karczewska-Kupczewska M, Kowalska I, Nikolajuk A, Adamska A, Otziomek E, Gorska M, Straczkowski M. Hyperinsulinemia acutely increases serum macrophage inhibitory cytokine-1 concentration in anorexia nervosa and obesity. Clin Endocrinol (Oxf). 2012; 76:46–50.
37. Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006; 236:13–23.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download