Abstract
Background
The effect of livingstone potato (Plectranthus esculenthus N.E.Br) on diabetes and its complications in Streptozotocin induced diabetic rats was investigated. The duration of the experiment was 4 weeks.
Methods
The blood glucose level of the rats was measured with a glucometer, the protein and glucose and specific gravity in the urine samples of the rats were measured using urine assay strips and urinometer respectively. The liver and kidney function parameters in the serum of the rats were determined using Biosystem Kits.
Results
The diabetic rats given livingstonepotato incorporated feeds, had 129.7% decrease in their hyperglycemia with corresponding amelioration of their elevated urinary protein, sugars, specific gravity, renal growth, liver growth as well as 15.64% decrease in body weights compared with the nondiabetic rats that had 5.54% decrease in blood glucose and 20.39% increase in body weight unlike the diabetic control rats that had 18.34% decrease in blood glucose and 52.68% decrease in body weight. There were significant differences (P<0.05) in the relative liver, pancreas, and kidney weights of the diabetic rats given livingstone potato feeds compared with the diabetic control while there were no significant differences (P>0.05) in the relative heart weights of all the rats in the three different groups. In terms of liver and kidney function parameters, values obtained for the diabetic rats given livingstone potato incorporated feeds were not significantly different from that of the nondiabetic rats except for total bilurubin, aspartate transaminase, and creatinine (P>0.05) while they were significantly different from the values obtained for the diabetic control rats (P<0.05). In addition, the serum amylase of the diabetic control rats were significantly higher (P<0.05) than that of the nondiabetic and diabetic rats treated with livingstone potato incorporated feeds.
Diabetes mellitus is one of the most challenging metabolic diseases of the 21st century which prevalence is rising globally. One of the major complications associated with diabetes mellitus is the alteration in the functions of the liver and renal systems [1].
The use of medicinal plants in the traditional management of diabetes mellitus has continued to play important roles in the lives of rural people, particularly those in the remote parts of developing countries that have limited access to adequate health facilities.
In many countries of the world, much attention has been paid to find novel types of natural antidiabetic drugs from various medicinal plants. Due to the effectiveness, limited side effects and relatively low cost of herbal drugs, they are widely prescribed even when their biologically active compounds are unknown [2].
Livingstone potato (Plectranthus esculenthus N.E.Br) which is known by its local name in Nigeria as rizga, is one of the widely cultivated minor root crops in the middle belt regions especially Kaduna and Plateau States of Nigeria for its finger like tubers [3]. The plant is also commonly found in Southern Africa, Malawi, Zimbabwe, Congo, Zambia, and Asia [4].
In terms of protein content, when compared with cassava, yam, cocoyam, and potato, it ranks highest [3]. Despite its nutritive potential, it is classified amongst the lesser known and underexploited species of root crops in Africa [3].
Livingstone potato is used in ethnopharmacology in Africa in the treatment of digestive problems [4], stomach ache [5], pains [6], and cancer. In the Northern parts of Nigeria, it is eaten mostly as snacks, porridge, fried, or cooked in stews [4,7] while in some other parts of Africa, it is used as a food additive [6].
In our previous investigations, we reported that the plant contains considerable antioxidant activity as well as phenolic phytochemicals-alkaloids, flavonoids, and tannins [8].
Since antioxidants could prevent the progressive impairment of pancreatic beta cell function due to oxidative stress, thereby reducing the occurrence of diabetes and being that the inhibition of the glycolytic activity of brush border enzymes by polyphenolic compounds is one of the factors that stimulates hypoglycemic action in some medicinal plants [9], we decided to commence a preliminary investigation for the first time on the possibilities of using this underexploited root crop in the management of diabetes and its complications.
The Plectranthus esculenta varieties were obtained at harvest from National Root Crops Research Institute (NRCRI), Umudike, Nigeria. They were identified by NRCRI, Umudike that has livingstone potato as a National Mandate as well as by a Taxonomist in Michael Okpara University of Agriculture, Umudike, Nigeria and deposited in their herbarium for authentication.
Streptozotocin (STZ) used was product of Sigma and Aldrich Chemical Company, United Kingdom. The creatinine, bilirubin, total protein, albumin, and amylase assay kits used were purchased from Biosystems, Barcelona, Spain.
The samples were properly washed, chopped and oven dried at 60℃ for 24 hours until constant weight was obtained. The dry samples were then processed to flour and incorporated into the standard rat feeds.
Male albino rats of the wistar strain (140 to 208 g) obtained from the animal house of the Department of Biochemistry, University of Nigeria, Nsukka, Enugu State, Nigeria, were used for the study. The animals were kept in metabolic cages in the animal house of the Department of Biochemistry, Michael Okpara University of Agriculture, Umudike, Nigeria. The animals were acclimatized for two weeks to their diets prior to the commencement of the experiment and were maintained under a constant 12-hour light and dark cycle and at room temperature. The National Institutes of Health Principles of Laboratory Animal Care were observed [10].
Freshly prepared solution of STZ (0.1 g dissolved in 5 mL of freshly prepared sodium citrate buffer 0.1 M, pH 4.5) was injected intraperitoneally to the rats at a dosage of 65 mg/kg body weight at fasting state. Blood was collected from the tail vein and the blood glucose concentration was analyzed prior to the commencement of the dietary feeding using a blood glucose meter (Double G glucometer, USA) and subsequently, twice in a week, throughout the duration of the experiment. The STZ-treated rats with fasting blood glucose (FBG) levels >200 mg/dL after 12 days of induction of STZ and evidence of glycosuria, were considered to be diabetic and were used for the study. The severity of diabetes was checked in the 24-hour urine samples of the STZ-treated rats using Urine Glucose Detection Strips (Clinistix; Bayer Health Care, Whippany, NJ, USA) and Urine Reagent Strips for urinalysis (qualitative and quantitative) tests for glucose and protein (CONDOR-TECHO URS-10; Condor Teco Medical Technology Co., Beijing, China). The specific gravity of the urine samples was determined with a urinometer. The animals were also observed for physical activity such as excessive thirst (polydypsia) and excessive hunger (polyphagia).
The experimental rats with stable diabetic condition were then divided into two subgroups (groups 2 and 3) while the nondiabetic group formed the first group as follows: group 1, normal rats administered standard rat feeds (nondiabetic control); group 2, diabetic control rats which also received standard rat feeds; group 3, diabetic rats treated with livingstone potato incorporated feeds (19.55% incorporation).
Their diets and water were both administered ad libitum for 28 days, after which the rats were stunned by blow, sacrificed and blood was collected intravenously from their heart using 10 mL syringes. The liver, kidney, and heart were also collected and weighed. The body weights of the rats were recorded on a daily basis, using an electronic weighing balance (Model Scout Pro; Ohaus Corp., Parsippany, NJ, USA) and the percentage change in weight was calculated as:
The serum aspartate transaminase (AST) and alanine transaminase (ALT) levels of the rats were determined spectrophotometrically at 540 nm using the Biosystems diagnostic kits as described by Gella et al. [11]. The total and direct (conjugated) bilirubin levels of the rats were determined using the Biosystems diagnostic kits and the principle was based on the reaction of the serum bilirubin with diazo reagent to form a coloured complex which is measured at 540 nm with a spectrophotometer spectrophotometer [12]. The serum creatinine of the rats were determined using the Biosystems kit and the principle was based on the reaction of the creatinine in the sample with picrate in alkaline medium to form a colored complex which is measured spectrophotometrically at 500 nm. The serum amylase, serum lipase, total proteins, albumin, and urea levels of the rats were also determined using Biosystems diagnostic kits using the methods described by previous researchers [12,13,14].
Data was subjected to analysis using the SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). Results were presented as the means±standard deviations of triplicate experiments. One way analysis of variance was used for comparison of the means. Differences between means were considered to be significant at P<0.05 using the New Duncan multiple range test.
The administration of STZ at a dosage of 65 mg/kg body weight to the rats of group 2 and 3, produced stable diabetic condition within 12 days in most of the experimental rats. Intake of the livingstone potato incorporated feeds to the diabetic rats of group 3, resulted in 129.7% decrease in their hyperglycemia by the last week of the experimentation, compared with the diabetic control rats that recorded 18.34% increase in blood glucose and the nondiabetic rats that recorded 5.54% decrease in their blood glucose level (Table 1).
The diabetic rats of groups 2 and 3 had elevated levels of glucose, specific gravity, and protein in their urine samples by the 1st and 2nd weeks of the experimentation (Table 2). However, by the last week of the experimentation, intake of livingstone potato incorporated feed to the diabetic rats of group 2, resulted in decreased levels of these parameters in their urine samples.
The diabetic control rats had significant increase of their relative liver and kidney weights (P<0.05) but significant decrease of their relative pancreatic weight (P<0.05) compared with the nondiabetic rats (Table 3). However, the diabetic rats fed livingstone potato incorporated feeds had significant reduction (P<0.05) of their relative liver and kidney weights but significant increase of their relative pancreatic weight (P<0.05) compared with the diabetic control rats. There were no significant differences (P>0.05) in the relative heart weight of all the rats in the three different groups (Table 3).
The diabetic rats fed livingstone potato incorporated feeds recorded 15.64% decrease in body weights, compared with diabetic control rats that recorded 52.68% decrease in body weights unlike the nondiabetic rats that recorded 20.39% increase in body weights (Table 4).
The serum conjugated bilirubin, total bilirubin, AST, and ALT levels of the diabetic control rats were significantly (P<0.05) higher than those of the nondiabetic rats while these parameters were significantly reduced in the serum of the diabetic rats given livingstone potato incorporated feeds compared with the diabetic control rats (Table 5).
The diabetic control rats had significantly reduced (P<0.05) levels of serum total protein and albumin but significantly elevated (P<0.05) levels of serum creatinine and urea in comparison with the nondiabetic rats. Intake of livingstone potato incorporated feeds by the diabetic rats of group 3, resulted in significant elevation (P<0.05) of their serum total protein and albumin but significant reduction (P<0.05) of their serum creatinine and urea levels compared with the diabetic control rats (Table 6).
The serum amylase levels of the diabetic control rats were significantly decreased (P<0.05) compared with the nondiabetic rats (Fig. 1). Furthermore, the serum amylase levels of the diabetic rats given livingstone potato incorporated feeds were significantly higher (P<0.05) than those of the diabetic control rats but not significantly different (P>0.05) from those of the nondiabetic rats. The serum lipase levels of the diabetic control rats were significantly higher (P<0.05) than those of the diabetic control rats (Fig. 1). Intake of livingstone potato incorporated feeds by the diabetic rats of group 3, resulted in significant decreases of their serum lipase levels compared with the diabetic control rats.
The STZ rat model of diabetes is one of the most commonly used models of human disease [15] because it mimics many of the complications of human diabetes apart from being reproducible. Results obtained in this study with respect to blood glucose, show the ability to livingstone potato to ameliorate hyperglycemia in diabetics.
The excretion of large amounts of glucose in the urine samples (glucosuria) of the STZ administered rats was an indication that their renal threshold of glucose was exceeded.
The glomerular membrane permits only very small amount of plasma proteins and values greater than 30 mg/dL may be indicative of significant proteinuria [16]. The development of diabetic nephropathy is characterized by a progressive increase in urinary protein and a late decline in glomerular filtration rate, leading to end stage renal failure [17]. The pathophysiology involves glucose that binds irreversibly to proteins in the kidney and circulation to form advanced glycosylation end products (AGEs). These AGEs can contribute to renal damage by the formation of growth and fibrotic factors through the receptors for AGEs. Increased glomerular capillary pressure occurs early in diabetes and is associated with hyperfiltration at the glomerulus. The glomerular messangium expands, initially by cell proliferation and then by cell hypertrophy. Thus the trace amount of proteins in the urine samples of the diabetic rats administered livingstone potato incorporated feeds, by the last week of the experiment, suggest the ability of livingstone potato to ameliorate glomerular complication in diabetics.
Specific gravity is a urinalysis parameter that helps in the evaluation of kidney function and diagnosis of renal diseases. Twenty four hour urine from normal patients may vary from 1.003 to 1.04 [9] while the specific gravity of rats varies from 1.022 to 1.05 [18]. The elevated levels of specific gravity in the urine samples of the diabetic control rats and the diabetic rats administered livingstone potato incorporated feeds by the first week of experimentation, compared with the nondiabetic rats, could be attributed to their elevated levels of urinary glucose as well as proteins and shows in addition, the possibilities of other substances that may have permeated the membrane of the glomerular filtrate as a result of renal complications. However, the reduction in the elevated urinary specific gravity of the diabetic rats administered livingstone potato incorporated feeds by the third and last week of the experimentation, indicates the ability of livingstone potato to ameliorate renal complication.
The significant increase in the relative kidney weights of diabetic control rats compared with the nondiabetic rats and the diabetic rats given livingstone potato incorporated feeds, is suggestive of diabetic glomerular hypertrophy which constitutes an early event in the progression of glomerular pathology [19] while the significant decrease (P<0.05) in the relative kidney weights of the diabetic rats given livingstone potato incorporated feeds, compared with the diabetic control rats and which decrease was not significantly different (P>0.05) from that of the nondiabetic rats, indicate the ability of livingstone potato to ameliorate diabetic glomerular hypertrophy.
The increase in the relative liver weight of the diabetic control rats could be attributed to triglyceride accumulation in the liver, leading to enlarged liver (hypertrophy). Results obtained with respect to diabetes and hypertrophy, are in agreement with earlier reports of Habibuddin et al. [20], Lee et al. [21], and Merzouk et al. [22] while the decrease in the relative liver weights of the diabetic rats given livingstone potato incorporated feeds indicates the ameliorating actions of livingstone potato on liver hypertrophy.
The decrease in the relative weight of the pancreas of the diabetic control rats compared with the diabetic rats givend livingstone potato incorporated feeds, is attributed to the disruption and disappearance of pancreatic islets and selective destruction of insulin-producing cells [23,24].
One plausible explanation for the toxic action of STZ on the liver, kidney, and pancreas of the diabetic control rats is their expression of GLUT2 transporter. GLUT2 is primarily expressed in the hepatocytes and pancreatic β-cells with lower levels expressed in the kidney, intestines, and retina [25]. Cells that express this GLUT2 transporter are susceptible to STZ attack [26,27]. Going by what we observed in this study regarding renal growth, one possible mechanism of action that we predict for the amelioration of renal growth by livingstone potato, is the inhibition of the formation of AGEs that contribute to renal damage by the formation of growth and fibrotic factors through their receptors as earlier mentioned.
The nonsignificant increase in the relative heart weights of the diabetic control rats compared with the nondiabetic rats and diabetic rats given livingstone potato incorporated feeds, can be attributed to the nonsusceptibility of the heart to STZ attack as the heart expresses GLUT4 transporter [28].
The decrease in the body weight of the diabetic control rats can be explained on the basis of increased muscle wasting and loss of tissue proteins [29]. The increased body weight in the diabetic rats given livingstone potato incorporated feeds compared with the diabetic control rats, is indicative of the protective action of livingstone potato against muscle wasting and synthesis of proteins. It could also indicate improvement in insulin secretion as insulin is the major regulator of glycogenolysis in the muscle and liver [29].
Bilirubin is excreted by the liver and any interference with the normal liver function affects its rate of conjugation and excretion. Therefore, a high level of bilirubin is used as an index of measurement of liver function and bile excretion status [30]. Thus the significant increase (P<0.05) in the levels of the total and conjugated bilirubin in the diabetic control rats indicates defect in the normal liver function of the rats of this group while the significant reduction in the total and conjugated bilirubin levels of the diabetic rats administered livingstone potato incorporated feeds, suggests the ability of livingstone potato to enhance liver function in diabetics.
The intracellular enzyme, ALT is a better marker of liver disease than AST as it increases in all kinds of liver diseases unlike AST that increases in both liver, myocardial infarction and heart muscle diseases [31,32]. STZ induces hepatocellular damage to all tissues that express the GLUT2 transporters such as the liver cells as earlier mentioned and this explains the higher serum levels of AST and ALT in the diabetic control rats compared with other groups investigated. The release of these enzymes into the serum is as a result of tissue injury or alterations in the permeability of the liver membrane [33]. The significant reduction in the transaminase levels of the diabetic rats administered livingstone potato incorporated feeds suggests that livingstone potato contains active compounds that are capable of ameliorating liver damage.
Amylase helps in the digestion of starch in the small intestine by acting on α-1-4-glycosidic linkages of starch, converting them to maltose which is further hydrolyzed to glucose moieties that are absorbed in the small intestine and transported to the blood stream [31]. Previous studies showed that amylase activity was significantly decreased in STZ diabetic rat models [34,35,36,37,38]. This decrease in the α-amylase activity of the diabetic control rats as observed in our study, is attributed to the inhibition of STZ on Ca and Mg homeostasis and amylase gene expression [35]. The observed increase in the serum amylase activity of the diabetic rats administered livingstone potato incorporated feeds could be as a result of improvement in insulin secretion since insulin enhances pancreatic amylase synthesis by enhancing its mRNA level [35].
Pancreatic lipase, a crucial enzyme in lipid metabolism, is a more specific marker of pancreatic dysfunction than pancreatic amylase [31] and it has also been employed in human and animal model studies involving the evaluation of natural products for their usefulness as antiobesity and antidiabetic agents [39]. The reduced lipase activity of the diabetic rats administered livingstone potato incorporated feeds, is another indication of improvement in insulin action since insulin inhibits the activity of pancreatic lipase [38]. The inhibition of this enzyme significantly decreases the digestion and uptake of lipids, thereby decreasing the level of postprandial blood glucose in noninsulin-dependent diabetic patients.
Results obtained in this study indicate the ameliorating potentials of livingstone potato on pancreatic dysfunction and also shows in addition, the usefulness of livingstone potato in the prevention/management of dyslipidemia which is one of the leading causes of diabetic complications.
The biochemical basis of the antidiabetic actions of the Nigerian livingstone potato investigated can be explained from two perspectives. First, judging from its ability to lower the pancreatic lipase levels of the diabetic rats shows its ability to ameliorate pancreatitis that is implicated in the development of diabetes. Secondly, livingstone potato was reported to contain significant quantities of crude fibre (approximately 5.99%) [7]. Dietary fibre inhibits the activity of pancreatic lipase [40], which is important in the management of diabetes and obesity. Finally, livingstone potato has been reported to contain considerable levels of polyphenolic compounds-tannins, saponins, alkaloids, flavonoids, and tannins [7]. Tannins inhibit the activity of digestive enzymes such as trypsin. In addition, flavonoids, as antioxidants may prevent the progressive impairment of pancreatic β-cell function due to oxidative stress, thereby reducing the occurrence of diabetes. Thus it's plausible to infer that these crude fibre and phenolic-phytochemicals function in synergy to confer anti-diabetic potentials to this plant.
In conclusion, results obtained in this study, indicate the antidiabetic actions of livingstone potato and its ability to ameliorate glomerular complication and liver hypertrophy in diabetics. It is hoped that the findings of this study will be included in the pharmacological properties of this underexploited root crop which could help to increase its awareness.
Figures and Tables
Fig. 1
Serum amylase and lipase levels of nondiabetic, diabetic control, and diabetic rats administered livingstone potato (rizga) incorporated feeds. a,b,cMeans with different superscripts for each enzyme are significantly different (P<0.05).

References
1. Akpan OU, Ewa ID, Etim BE. Ocimum gratissimum alleviates derangements in serum and biliary bilirubin, cholesterol and electrolytes in streptozotocin-induced diabetic rats. Int J Biochem Res Review. 2013; 3:171–189.
2. Oche O, Sani I, Chilaka NG, Samuel NU, Samuel A. Pancreatic islet regeneration and some liver biochemical parameters of leaf extracts of Vitex doniana in normal and streptozotocin-induced diabetic albino rats. Asian Pac J Trop Biomed. 2014; 4:124–130.
3. Schippers RR. Natural Resources Institute (Great Britain). African indigenous vegetables: an overview of the cultivated species. Chatham: University of Greenwich, Natural Resources Institute;2000.
4. Burkill HM. The useful plants of West Tropical Africa. Kew: Royal Botanic Gardens;1995. Vol. 3:p. 857.
5. Pakia M, Cooke JA. The ethnobotany of the Midzichenda tribes of the coastal forest areas in Kenya: 2. Medicinal plant uses. S Afr J Bot. 2003; 69:382–395.
6. Lukhoba CW, Simmonds MS, Paton AJ. Plectranthus: a review of ethnobotanical uses. J Ethnopharmacol. 2006; 103:1–24.
7. Temple VJ, Onobun CE, Ojobe TO. Chemical composition of livingstone potato tubers (Plectranthus esculentus). J Sci Food Agric. 1991; 56:215–217.
8. Eleazu CO, Eleazu KC, Ikpeama AI. Phenolic content, antioxidant capacity and toxicity of 3 varieties of living stone potato (Rizga). J Pharmacol Toxicol. 2012; 7:206–212.
9. Eleazu CO, Iroaganachi M, Eleazu KC. Ameliorative potentials of cocoyam (Colocasia esculenta L.) and unripe plantain (Musa paradisiaca L.) on the relative tissue weights of streptozotocin-induced diabetic rats. J Diabetes Res. 2013; 2013:160964.
10. National Research Council (États-Unis) Committee on Care and Use of Laboratory Animals. National Institutes of Health (États-Unis) Animal Resources Program Branch. Guide for the care and use of laboratory animals. Bethesda: Animal Resource Program, Division of Research Resources, National Institutes of Health;1985.
11. Gella FJ, Olivella T, Cruz Pastor M, Arenas J, Moreno R, Durban R, Gomez JA. A simple procedure for the routine determination of aspartate aminotransferase and alanine aminotransferase with pyridoxal phosphate. Clin Chim Acta. 1985; 153:241–247.
12. Burtis CA, Ashwood ER, Tietz NW. Tietz textbook of clinical chemistry. 3rd ed. Philadelphia: W.B. Saunders;1999.
13. Friedman RB, Young DS. Effects of disease on clinical laboratory tests. 3rd ed. Washington, DC: AACC Press;1997.
14. Young DS. Effects of drugs on clinical laboratory tests. 3rd ed. Washington, DC: AACC Press;1990.
15. Ugarte M, Brown M, Hollywood KA, Cooper GJ, Bishop PN, Dunn WB. Metabolomic analysis of rat serum in streptozotocin-induced diabetes and after treatment with oral triethylenetetramine (TETA). Genome Med. 2012; 4:35.
16. Deb AC. Concepts of Biochemistry. 2nd ed. Kolkata: Books and Allied Ltd.;2006.
17. Soleimani S, Fathiazarbaijani F, Nejati V, Shojaei SH, Nanghshbandi N. Effect of Equisetum arvense L. (Equisetaceae) in microalbuminuria and creatinine excretion in streptozotocin-induced diabetes in male rats. Int J Pharmacol. 2007; 3:155–159.
18. Johnson-Delaney CA, Harrison LR. Exotic companion medicine handbook for veterinarians. Lake Worth: Wingers Pub;1996.
19. Malatiali S, Francis I, Barac-Nieto M. Phlorizin prevents glomerular hyperfiltration but not hypertrophy in diabetic rats. Exp Diabetes Res. 2008; 2008:305403.
20. Habibuddin M, Daghriri HA, Humaira T, Al Qahtani MS, Hefzi AA. Antidiabetic effect of alcoholic extract of Caralluma sinaica L. on streptozotocin-induced diabetic rabbits. J Ethnopharmacol. 2008; 117:215–220.
21. Lee SI, Kim JS, Oh SH, Park KY, Lee HG, Kim SD. Antihyperglycemic effect of Fomitopsis pinicola extracts in streptozotocin-induced diabetic rats. J Med Food. 2008; 11:518–524.
22. Merzouk H, Madani S, Chabane Sari D, Prost J, Bouchenak M, Belleville J. Time course of changes in serum glucose, insulin, lipids and tissue lipase activities in macrosomic offspring of rats with streptozotocin-induced diabetes. Clin Sci (Lond). 2000; 98:21–30.
23. Kim JD, Kang SM, Seo BI, Choi HY, Choi HS, Ku SK. Anti-diabetic activity of SMK001, a poly herbal formula in streptozotocin induced diabetic rats: therapeutic study. Biol Pharm Bull. 2006; 29:477–482.
24. Heidari Z, Mahmoudzadeh-Sagheb H, Moudi B. A quantitative study of sodium tungstate protective effect on pancreatic beta cells in streptozotocin-induced diabetic rats. Micron. 2008; 39:1300–1305.
25. Gould GW, Thomas HM, Jess TJ, Bell GI. Expression of human glucose transporters in Xenopus oocytes: kinetic characterization and substrate specificities of the erythrocyte, liver, and brain isoforms. Biochemistry. 1991; 30:5139–5145.
26. Elsner M, Guldbakke B, Tiedge M, Munday R, Lenzen S. Relative importance of transport and alkylation for pancreatic beta-cell toxicity of streptozotocin. Diabetologia. 2000; 43:1528–1533.
27. Medina RA, Owen GI. Glucose transporters: expression, regulation and cancer. Biol Res. 2002; 35:9–26.
28. Bellamkonda R, Rasineni K, Singareddy SR, Kasetti RB, Pasurla R, Chippada AR, Desireddy S. Antihyperglycemic and antioxidant activities of alcoholic extract of Commiphora mukul gum resin in streptozotocin induced diabetic rats. Pathophysiology. 2011; 18:255–261.
29. Usha K, Kasturi GM, Hemalatha P. Hepatoprotective effect of Hygrophila spinosa and Cassia occidentalis on carbon tetrachloride induced liver damage in experimental rats. Indian J Clin Biochem. 2007; 22:132–135.
30. Nayak SB. Manipal manual of clinical biochemistry: for medical laboratory MSc students. 3rd ed. New Delhi: Jaypee Brothers Medical Publishers;2007.
31. Saxena A. Chapter, Fundamentals of Biochemistry. Textbook of biochemistry. New Dehli: Discovery;2006. p. 56–57.
32. Hsueh CJ, Wang JH, Dai L, Liu CC. Determination of alanine aminotransferase with an electrochemical nano Ir-C biosensor for the screening of liver diseases. Biosensors. 2011; 1:107–117.
33. Gupta R, Katariya P, Mathur M, Bajaj VK, Yadav S, Kamal R, Gupta RS. Anti-diabetic and renoprotective activity of momordica dioica in diabetic rats. Diabetol Croatica. 2011; 40:81.
34. Patel R, Yago MD, Manas M, Victoria EM, Shervington A, Singh J. Mechanism of exocrine pancreatic insufficiency in streptozotocin-induced diabetes mellitus in rat: effect of cholecystokinin-octapeptide. Mol Cell Biochem. 2004; 261:83–89.
35. Omoruyi FO, Budiaman A, Eng Y, Olumese FE, Hoesel JL, Ejilemele A, Okorodudu AO. The potential benefits and adverse effects of phytic acid supplement in streptozotocin-induced diabetic rats. Adv Pharmacol Sci. 2013; 2013:172494.
36. Emam MA. Comparative evaluation of antidiabetic activity of Rosmarinus officinalis L. and Chamomile recutita in streptozotocin induced diabetic rats. Agric Biol J North Am. 2012; 3:247.
37. Aughsteen AA, Mohammed FI. Insulin enhances amylase and lipase activity in the pancreas of streptozotocin-diabetic rats. An in vivo study. Saudi Med J. 2002; 23:838–844.
38. Aloulou A, Hamden K, Elloumi D, Ali MB, Hargafi K, Jaouadi B, Ayadi F, Elfeki A, Ammar E. Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats. BMC Complement Altern Med. 2012; 12:63.
39. Lairon D, Lafont H, Vigne JL, Nalbone G, Leonardi J, Hauton JC. Effects of dietary fibers and cholestyramine on the activity of pancreatic lipase in vitro. Am J Clin Nutr. 1985; 42:629–638.
40. Dunaif G, Schneeman BO. The effect of dietary fiber on human pancreatic enzyme activity in vitro. Am J Clin Nutr. 1981; 34:1034–1035.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


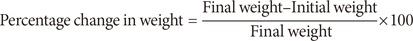
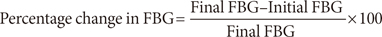
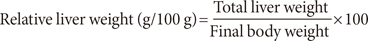
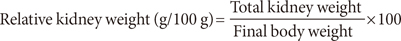
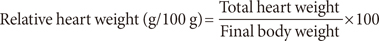






 XML Download
XML Download