Abstract
Background
Peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist has a wide-ranging influence on multiple components of metabolic syndrome. The Otsuka Long-Evans Tokushima Fatty (OLETF) rat is a useful animal model of metabolic syndrome. To determine genes related to metabolic syndrome, we examined overlapping genes that are simultaneously decreased by PPAR-γ agonists and increased in OLETF rats using microarrays in two different models.
Methods
In the first microarray analysis, PPAR-γ agonist-treated db/db mice were compared to standard diet-fed db/db mice. In the second microarray analysis, OLETF rats were compared to Long-Evans Tokushima Otsuka (LETO) rats (control of OLETF rats).
Results
Among the overlapping genes, in the present study, we validated that lipocalin-2 expression was significantly decreased in the visceral adipose tissue of PPAR-γ agonist-treated db/db mice compared to standard diet-fed db/db mice and increased in OLETF rats compared to LETO rats using real time reverse transcription polymerase chain reaction. Furthermore, we showed for the first time that lipocalin-2 expression was significantly increased in the visceral adipose tissues of obese humans compared with nonobese humans. In addition, the expression level of lipocalin-2 in human visceral adipose tissue had a significant positive correlation with body mass index, serum interleukin-6, adipocyte fatty acid binding protein levels, and white blood cell count.
Conclusion
Lipocalin-2 was confirmed to be a significant adipokine affected by PPAR-γ agonist and obesity in the present study. Also, for the first time in human visceral adipose tissue, it was determined that the expression of lipocalin-2 from obese humans was significantly increased and correlated with circulating inflammatory markers.
Peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist plays a key role in the regulation of adipose tissue function and has a significant effect on multiple components of metabolic syndrome [1]. Thiazolidinedione (TZD), a synthetic PPAR-γ agonist, favorably influences the production of adipokines and redistributes fat within the body, thereby contributing to an improvement in insulin sensitivity [2]. In addition, several animal and human studies have shown that TZD treatment is associated with usually small but significant reductions in blood pressure [3,4,5]. Moreover, TZD is known to increase high density lipoprotein cholesterol significantly and to decrease triglycerides, which are major contributors to metabolic syndrome [6]. Therefore, considerable research efforts have investigated novel genes regulated by PPAR-γ to determine genes related to metabolic syndrome.
The Otsuka Long-Evans Tokushima Fatty (OLETF) rat is a useful animal model of metabolic syndrome, as these rats are characterized by abdominal obesity, insulin resistance, hypertension, and dyslipidemia [7]. OLETF has a control counterpart animal model, the Long-Evans Tokushima Otsuka (LETO) rat, which was developed from the same colony but does not develop insulin resistance and obesity [8]. These two strains have been used in a number of studies to clarify the pathogenesis of metabolic syndrome [9,10,11].
Microarrays have provided medical researchers with a powerful tool to study the mechanisms of complex diseases, including type 2 diabetes and obesity [12]. However, there are still numerous problems and challenges in the current application of microarrays [13,14]. In particular, a vast number of genes are usually detected using microarrays, requiring a laborious validation process [15]. Therefore, in this study, we performed microarrays in two different models and searched overlapping genes to identify genes that are significant multiple conditions and thus reduce the number of identified genes requiring further validation.
We primarily investigated lipocalin-2, which has been recently re-evaluated as a novel adipokine correlated with obesity-related metabolic disorders [16]. Recently, Wang et al. [16] exhibited circulating lipocalin-2 levels and the expression of lipocalin-2 in adipose tissue, which were increased in db/db obese diabetic mice compared with normal mice. Furthermore, serum lipocalin-2 levels were elevated in obese compared to lean humans and correlated with various components of the metabolic syndrome [16]. However, there have been no studies examining the difference of lipocalin-2 expression in direct human adipose tissue in obese and nonobese. No study has examined the relationship of lipocalin-2 expression in human adipose tissue with circulating metabolic parameters. Therefore, in this study, we planned to confirm the significance of lipocalin-2 using microarrays in two different rodent models, and we intended to validate, for the first time, the difference of lipocalin-2 expression in adipose tissue between obese and nonobese humans.
We examined the adipose gene expression profiles, which were regulated by PPAR-γ agonist treatment in db/db mice, a rodent model of metabolic syndrome using microarray analysis. Additionally, in the second microarray analysis, another set of adipose gene expression profiles, which were differentially expressed in OLETF rats compared with LETO rats, were searched. Finally, to determine the important genes related to metabolic syndrome, we examined overlapping genes whose expression is decreased by PPAR-γ agonist treatment and increased in OLETF rats using microarrays in two different models, and we validated the significant overlapping genes in independent rodent and human adipose tissue samples (Fig. 1).
Five-week-old male db/db mice (SLC, Shizuoka, Japan), which have a mutation in the leptin receptor, are used as an animal genetic model of metabolic syndrome [17]. The mice were acclimated to standard chow (AIN-93G; Dyets, Bethlehem, PA, USA) for 1 week, after which they were weighed and divided into two groups: (1) a group on a standard diet (n=5); and (2) a group on a standard diet mixed with pioglitazone (Takeda Pharmaceutical, Osaka, Japan), a synthetic PPAR-γ agonist (n=5). Pioglitazone was administered to 6-week-old db/db mice for 10 days at a dose of 40 mg/kg/day. At the end of the study period, the animals were sacrificed and the visceral adipose tissue (mesenteric depots) was harvested.
Four-week-old male OLETF rats (70 to 80 g initial weight) were supplied from Otsuka Pharmaceutical (Tokushima, Japan). Age-matched LETO rats were used as normal controls. At 24 weeks of age, OLETF (n=5) and LETO rats (n=5) were sacrificed and their visceral adipose tissues (mesenteric depots) were harvested. All experiments were conducted in accordance with the Korea University Guidelines for the Care and Use of Experimental Animals.
Total RNA from visceral adipose tissues of pioglitazone-treated db/db mice (n=5), normal chow-fed db/db mice (n=5), OLETF rats (n=5), and LETO rats (n=5) was extracted using an RNeasy Mini kit according to the manufacturer's instructions (Qiagen, Baltimore, MD, USA). Using a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA), the quality of extracted RNA was analyzed, and microarray analyses in two different models were performed: (1) between pioglitazone-treated db/db mice and normal chow-fed db/db mice; and (2) between OLETF rats and LETO rats. For control and test RNAs, the synthesis of target cRNA probes and hybridization were performed using a Low RNA Input Linear Amplification kit (Agilent Technologies) according to the manufacturer's instructions. Total twelve samples (six samples per model) were used for microarray hybridizations. The arrays were hybridized at 65℃ for 17 hours using a hybridization oven (Agilent Technologies). DNA chips (Agilent mouse whole genome 251486813056, Agilent rat whole genome 251487913127) were scanned using Scan Array Lite (Perkin-Elmer Life Sciences, Billerica, MA, USA). Scanned images were analyzed with GenePix 3.0 software (Axon Instruments, Union City, CA, USA) to obtain gene expression ratios. Logged gene expression ratios were normalized by LOWESS regression.
Six premenopausal obese women (body mass index [BMI] >25 kg/m2) and six age-matched nonobese premenopausal women (BMI <23 kg/m2) who were scheduled for gynecological surgery (total abdominal hysterectomy or myomectomy for noninflammatory uterine leiomyoma) were enrolled in this study. Obesity was defined with BMI according to the Asia-Pacific criteria [18]. Exclusion criteria included pregnancy, heart disease, cancer, inflammatory conditions, diabetes, and any other endocrine or metabolic disorder. Women with a history of hormone replacement therapy or oral contraceptive use or who were taking medications within the previous 3 months that could affect their inflammatory condition were also excluded.
Weight and height were measured to calculate BMI (kg/m2). Total serum cholesterol was determined by enzymatic methods using a chemical analyzer (Hitachi 747; Hitachi, Tokyo, Japan). The glucose oxidase method was used to measure plasma glucose. Interleukin-6 (IL-6) levels were measured by a Quantikine kit (R&D Systems, Belmont, CA, USA) with a coefficient of variation (CV) of 8.1%. Adipocyte fatty acid binding protein (A-FABP) levels were measured by an enzyme-linked immunosorbent assay kit (BioVendor Laboratory Medicine, Brno, Czech Republic) with a CV of 3.11%, and lipocalin-2 was measured by a Quantikine kit (R&D System) with a CV of 1.43%.
Using a cold knife, small pieces (5 to 10 g wet weight) of subcutaneous adipose tissue were obtained from the abdominal incision site. In addition, visceral adipose tissue (mesenteric depots) was obtained during the gynecologic surgery. The adipose tissues were washed with sterile Krebs-Ringer-HEPES buffer and were stored at -70℃.
Double-stranded cDNA was synthesized using a High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA, USA). Expression levels of lipocalin-2 in the visceral adipose tissues of humans and rodents were analyzed by a quantitative real-time reverse transcription polymerase chain reaction (qRTRT-PCR). qRTRT-PCR analysis was conducted using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems) according to the manufacturer's instructions. TaqMan probes for lipocalin-2 (Mn00809552_s1, Hs00194353_m1, Rn00590612) and β-actin (Mm00607939_s1, Hs99999903_m1, Rn00667869) were the Assay-on-Demand gene expression products (Applied Biosystems). The mRNA levels of all genes were corrected using the transcription level of β-actin as an internal standard.
Values are presented as mean±standard deviation. Differences between the two groups were tested using the Mann-Whitney U test. One-sample Student t-test was calculated to test whether the mean normalized expression value for the gene was significantly different. A one sample binomial test was used to calculate whether there was a significant difference between the number of genes up-regulated and down-regulated by PPAR-γ agonist within each pathway. A Spearman correlation analysis was performed to determine the relationship between the level of lipocalin-2 expression in visceral adipose tissue and clinical and biochemical variables. A P<0.05 was considered to be statistically significant. Data were analyzed using SPSS version 10.0 (SPSS Inc., Chicago, IL, USA).
db/db mice fed with a pioglitazone mixed diet for 10 days showed a greater increase in body weight than those on a standard diet (P<0.01) (Table 1). Additionally, there was a significant decrease in serum fasting glucose and triglyceride levels with pioglitazone treatment (P<0.001 and P=0.027, respectively). The body weight of OLETF rats at 24 weeks was significantly increased compared with that of LETO rats (P=0.05), and there was a significant increase in serum fasting glucose, triglyceride, and total cholesterol levels in OLETF rats (Table 1).
To clarify which pathways were truly affected by PPAR-γ, we focused on differentially expressed genes using filtering criteria of at least 2.0-fold-changes in all the experiment sets with a false discovery rate <5% [19]. The predominant pathways that were differentially represented in the visceral adipose tissues of db/db mice after PPAR-γ agonist treatment are shown in Table 2. The KEGG public category with the highest number of differentially expressed genes in a positive direction was the oxidative phosphorylation pathway. The other three KEGG public pathways positively affected by PPAR-γ agonist were PPAR signaling, fatty acid metabolism, and the citrate cycle pathway, in that order. In contrast, the T-cell receptor signaling pathway was identified as the pathway most down-regulated by PPAR-γ agonist followed by cytokine-cytokine receptor interactions, natural killer cell-mediated cytotoxicity, and B-cell receptor signaling pathways.
Based on previous studies [16,20], we chose lipocalin-2 as a significant gene, which needed to be validated by real-time RT-PCR. Lipocalin-2 is a recently re-evaluated adipokine that is related to obesity related metabolic disorders [16]. Our research team has published that serum lipocalin-2 levels were significantly elevated in patients with coronary heart disease (CHD) and independently associated with CHD [20]. In the first microarray analysis, the average gene expression fold change of lipocalin-2 in PPAR-γ agonist treated db/db mice when divided by normal chow fed db/db mice was 0.39 (P=0.011). In the second microarray analysis, the average gene expression fold change of lipocalin-2 in OLETF rats divided by that of LETO rats was 1.59 (P=0.077).
Real-time RT-PCR validation studies in independent samples showed that there was a significant increase in lipocalin-2 expression in visceral adipose tissue of OLETF rats compared with LETO rats (P=0.01) (Fig. 2A), while the level of lipocalin-2 mRNA in visceral adipose tissue of pioglitazone-treated db/db mice was significantly decreased compared with that of normal chow-fed db/db mice (P<0.001) (Fig. 2B).
The clinical characteristics of the 12 women are shown in Table 3. The average BMI in the obese group was 29.6±3.5 kg/m2, whereas the average BMI in the nonobese group was 21.1±1.2 kg/m2 (P<0.001). In the obese group, there was a significant increase in serum glucose (P=0.05), WBC count (P=0.004), A-FABP (P=0.004), and lipocalin-2 (P=0.03) levels compared with the nonobese group.
The level of expression of lipocalin-2 in the visceral adipose tissues of the obese group was significantly increased compared with the nonobese group (P=0.01) (Fig. 2C). However, there was no significant difference in the expression of lipocalin-2 in subcutaneous adipose tissues between the two groups (P=0.20) (Fig. 2D). Next, we analyzed the relationship between the expression of lipocalin-2 in visceral adipose tissues with anthropometric and biochemical variables. As a result, the expression of lipocalin-2 in visceral adipose tissues was significantly correlated with BMI (r=0.68, P=0.015), serum IL-6 (r=0.59, P=0.044), A-FABP (r=0.60, P=0.04) levels, and WBC count (r=0.72, P=0.008) (Fig. 3). Lipocalin-2 expression in visceral adipose tissue showed the trends of positive correlation with serum lipocalin-2 levels, although the correlation was not significant (r=0.50, P=0.1). Additionally, the serum lipocalin-2 level was closely correlated with BMI (r=0.71, P=0.01), WBC (r=0.71, P=0.01), and A-FABP levels (r=0.74, P=0.006).
Using microarrays in two different models, we tried to search adipose gene expression profiles that were simultaneously increased in a rodent model of metabolic syndrome and decreased with PPAR-γ agonist treatment. In the present study, lipocalin-2 was validated as a significant metabolic syndrome-related adipokine in both rodent and human adipose samples. Furthermore, this study is the first to demonstrate that the level of expression of lipocalin-2 is significantly increased in visceral adipose tissue of obese humans compared with nonobese humans and that these levels have a close positive correlation with BMI, serum IL-6, A-FABP levels, and WBC counts.
Microarray analysis permits a more comprehensive understanding of the interaction between multiple genes simultaneously involved in pathological conditions [12]. For example, retinol binding protein 4 (RBP4) is an adipokine that is newly discovered using microarray analysis and is elevated in the adipose tissue of adipose-Glut4-/- mice [21]. In addition, using cDNA array chips, a screen for genes that are induced during adipocyte differentiation but down-regulated in mature adipocytes exposed to PPAR-γ agonist led to the discovery of a protein called resistin [22]. Although microarray analysis is a useful methodology for the detection of new genes that are involved in a variety of metabolic syndromes, a very large number of genes are usually detected necessitating a laborious validation process. Our study design was intended to search for overlapping genes between two separate microarray analyses to find more meaningful genes with metabolic syndrome, between pioglitazone-treated db/db mice and normal chow-fed db/db mice and between OLETF rats and LETO rats. The use of two different rodent models most likely reduced the risk of identifying false positive genes.
Among the overlapping genes, based on recent studies, we validated lipocalin-2 using independent rodent and human adipose samples in this study. As a result, we verified that lipocalin-2 is simultaneously down-regulated in pioglitazone treated db/db mice and up-regulated in OLETF rats. Lipocalin-2 (also known as neutrophil gelatinase-associated lipocalin) is a 25-kDa acute phase protein originally purified from human neutrophils [23], a member of the lipocalin family, and shares a common tertiary structure formed by segments termed lipocalin folds [24]. RBP-4, which has been recently re-evaluated as a novel adipokine involved in the pathogenesis of insulin resistance using DNA arrays in adipose Glut4-/- mice, is another well-known lipocalin family [21]. Recently, Wang et al. [16] evaluated the relevance of lipocalin-2 in obesity-related disorders both in mice and humans. These researchers reported that lipocalin-2 expression in adipose tissue and liver were significantly increased in db/db mice compared with lean littermates [16]. In addition, the researchers also found a significant positive correlation between serum lipocalin-2 levels and several variables associated with obesity-related metabolic disorders in humans. In this study, we confirmed that the serum concentration of lipocalin-2 was significantly increased in the obese group and closely correlated with BMI, WBC count, and serum A-FABP levels. Furthermore, we found for the first time that the expression of lipocalin-2 in visceral adipose tissues of humans has a positive correlation with BMI, WBC count, and serum IL-6 and A-FABP levels. We examined the correlation of circulating inflammatory markers not only with the serum lipocalin-2 level but also with lipocalin-2 expression in human adipose tissue. To date, there have been a small number of studies examining the lipocalin-2 expression in visceral adipose tissue of rodents and 3T3-L1 adipoctyes [16,25,26]. However, there have been no studies regarding the lipoclin-2 expression level in direct human adipose tissue. Therefore, the present study is the first to show the difference in the level of lipocalin-2 expression in the visceral adipose tissues in obese and nonobese humans.
If lipocalin-2 expression in visceral adipose tissue had predicted the circulating levels of that cytokine in the present study, positive correlation of lipocalin-2 expression in visceral adipose tissue with various metabolic parameters might be more meaningful. However, lipocalin-2 expression in visceral adipose tissue showed only the trends of positive correlation with serum lipocalin-2 level (r=0.50, P=0.1) in the present study, and significance is expected if the sample size increased. In this study, the expression of lipocalin-2 in visceral adipose tissue had a significant positive relationship with serum IL-6 levels and WBC count, which are representative biomarkers of the inflammatory process [27,28]. Metabolic syndrome is closely associated with systemic low-grade inflammation [29]. Recently, Zhang et al. [25] reported that the administration of lipocalin-2 to 3T3-L1 adipocytes attenuated the inflammatory effect of tumor necrosis factor-α and lipopolysaccharide-induced cytokine production in macrophages, suggesting that lipocalin-2 is a novel adipokine acting as an antagonist of inflammatory pathways. By contrast, Yan et al. [26] demonstrated that the forced reduction of lipocalin-2 in 3T3-L1 adipocytes improved insulin action and that exogenous lipocalin-2 promotes insulin resistance in cultured hepatocytes, suggesting that lipocalin-2 is an independent adipokine to accelerate obesity-related pathogenesis. Although the previous two studies had different suggestions regarding the action of lipocalin-2, it is clearly based on the results of these study and our study that lipocalin-2 mediates an important crossroad between the obesity-related inflammatory process and metabolic response.
The limitation of our cross-sectional study was that we could not clarify whether the increase in lipocalin-2 was a simple compensatory response of obesity or whether lipocalin-2 independently affects the pathogenesis of obesity-related disorders. Second, the sample size of human participants is too small. There is lack of data regarding visceral adiposity, such as waist circumference and abdominal fat, on computed tomography.
In conclusion, to determine the genes involved in metabolic syndrome, we tried to search overlapping genes whose expression were both decreased in PPAR-γ agonist treated db/db mice and increased in OLETF rats based on two independent microarray results. Among these overlapping genes, we confirmed that lipocalin-2 is a significant adipokine affected by PPAR-γ agonist and obesity. We newly validated that the lipocalin-2 expression of human adipose tissue is significantly increased in obese humans compared with nonobese humans and is significantly correlated with several circulating metabolic parameters. Further study is warranted to clarify the functional role of lipocalin-2 in the pathogenesis of metabolic syndrome.
Figures and Tables
Fig. 1
Flow sheet of microarrays in two different models and validation process. PPAR-γ, peroxisome proliferator-activated receptor-γ; OLETF, Otsuka Long-Evans Tokushima Fatty; LETO, Long-Evans Tokushima Otsuka; RT-PCR, reverse transcription polymerase chain reaction.
Fig. 2
Expression of lipocalin-2 in adipose tissue from independent rodent and human samples. (A) Visceral adipose tissues of 24-week-old male Long-Evans Tokushima Otsuka (LETO) rats (black bars) and Otsuka Long-Evans Tokushima Fatty (OLETF) rats (white bars). (B) Visceral adipose tissues of standard chow-fed db/db mice (black bars) and pioglitazone-treated db/db mice (white bars). (C) Visceral adipose tissues of nonobese women (black bars) and obese women (white bars). (D) Subcutaneous adipose tissues of nonobese women (black bars) and obese women (white bars). Gene expression levels were measured by real time reverse transcription polymerase chain reaction. NS, non significant. aP<0.05, bP<0.01.
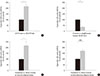
Fig. 3
Spearman correlation coefficient between the level of expression of lipocalin-2 in visceral adipose tissues of humans and (A) body mass index (BMI), (B) white blood cell (WBC) count, (C) serum interleukin-6 (IL-6), and (D) adipocyte fatty acid binding protein (A-FABP) levels. The horizontal axis of each graph indicates the expression ratio of lipocalin-2 versus β-actin in the visceral adipose tissues of humans measured by real time reverse transcription polymerase chain reaction.

Table 2
KEGG public pathway classification of differentially expressed genes in PPAR-γ agonist-treated db/db mice, as indicated by microarray data

Differentially expressed genes were selected using filtering criteria of at least 2.0-fold-changes in all the experimental sets with a false discovery rate <5%. "P value" indicates the significance of the difference in the number of up-regulated versus down-regulated genes by one sample binomial test.
KEGG, Kyoto Encyclopedia of Genes and Genomes; PPAR-γ, peroxisome proliferator-activated receptor-γ; MAPK, mitogen-activated protein kinase; TCA, tricarboxylic acid; VEGF, vascular endothelial growth factor.
ACKNOWLEDGMENTS
This research was supported by a grant (KMC) from the Korean Diabetes Association and from the Basic Science Research Program, National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (201006363)(KMC).
References
1. Staels B, Fruchart JC. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes. 2005; 54:2460–2470.
2. Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004; 89:463–478.
3. Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. PPAR(gamma) agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension. 2004; 43:661–666.
4. Raji A, Seely EW, Bekins SA, Williams GH, Simonson DC. Rosiglitazone improves insulin sensitivity and lowers blood pressure in hypertensive patients. Diabetes Care. 2003; 26:172–178.
5. Hirose H, Kawai T, Yamamoto Y, Taniyama M, Tomita M, Matsubara K, Okazaki Y, Ishii T, Oguma Y, Takei I, Saruta T. Effects of pioglitazone on metabolic parameters, body fat distribution, and serum adiponectin levels in Japanese male patients with type 2 diabetes. Metabolism. 2002; 51:314–317.
6. Elte JW, Blickle JF. Thiazolidinediones for the treatment of type 2 diabetes. Eur J Intern Med. 2007; 18:18–25.
7. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992; 41:1422–1428.
8. Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract. 1994; 24:Suppl. S317–S320.
9. Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, Hashimoto I, Okada T, Yasuhara A, Nakatsuka A, Shikata K, Hourai S, Futami J, Watanabe E, Matsuki Y, Hiramatsu R, Akagi S, Makino H, Kanwar YS. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci U S A. 2005; 102:10610–10615.
10. Chen D, Wang MW. Development and application of rodent models for type 2 diabetes. Diabetes Obes Metab. 2005; 7:307–317.
11. Shima K, Zhu M, Mizuno A. Pathoetiology and prevention of NIDDM lessons from the OLETF rat. J Med Invest. 1999; 46:121–129.
12. Sun G. Application of DNA microarrays in the study of human obesity and type 2 diabetes. OMICS. 2007; 11:25–40.
13. Draghici S, Khatri P, Eklund AC, Szallasi Z. Reliability and reproducibility issues in DNA microarray measurements. Trends Genet. 2006; 22:101–109.
14. Allison DB, Cui X, Page GP, Sabripour M. Microarray data analysis: from disarray to consolidation and consensus. Nat Rev Genet. 2006; 7:55–65.
15. Jayapal M, Melendez AJ. DNA microarray technology for target identification and validation. Clin Exp Pharmacol Physiol. 2006; 33:496–503.
16. Wang Y, Lam KS, Kraegen EW, Sweeney G, Zhang J, Tso AW, Chow WS, Wat NM, Xu JY, Hoo RL, Xu A. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. 2007; 53:34–41.
17. Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996; 84:491–495.
18. Bassett J. International Diabetes Institute. World Health Organization. Regional Office for the Western Pacific. International Association for the Study of Obesity. International Obesity Task Force. The Asia-Pacific perspective: redefining obesity and its treatment. Australia: Health Communications Australia;2000.
19. Gusnanto A, Calza S, Pawitan Y. Identification of differentially expressed genes and false discovery rate in microarray studies. Curr Opin Lipidol. 2007; 18:187–193.
20. Choi KM, Lee JS, Kim EJ, Baik SH, Seo HS, Choi DS, Oh DJ, Park CG. Implication of lipocalin-2 and visfatin levels in patients with coronary heart disease. Eur J Endocrinol. 2008; 158:203–207.
21. Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005; 436:356–362.
22. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001; 409:307–312.
23. Kjeldsen L, Cowland JB, Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim Biophys Acta. 2000; 1482:272–283.
24. Borregaard N, Cowland JB. Neutrophil gelatinase-associated lipocalin, a siderophore-binding eukaryotic protein. Biometals. 2006; 19:211–215.
25. Zhang J, Wu Y, Zhang Y, Leroith D, Bernlohr DA, Chen X. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol Endocrinol. 2008; 22:1416–1426.
26. Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z, Houstis NE, Kahn BB, Rosen ED. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007; 56:2533–2540.
27. Ruggiero C, Metter EJ, Cherubini A, Maggio M, Sen R, Najjar SS, Windham GB, Ble A, Senin U, Ferrucci L. White blood cell count and mortality in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2007; 49:1841–1850.
28. Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000; 101:1767–1772.
29. Das UN. Metabolic syndrome X: an inflammatory condition. Curr Hypertens Rep. 2004; 6:66–73.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download