Abstract
The pyruvate dehydrogenase complex (PDC) is an emerging target for the treatment of metabolic syndrome. To maintain a steady-state concentration of adenosine triphosphate during the feed-fast cycle, cells require efficient utilization of fatty acid and glucose, which is controlled by the PDC. The PDC converts pyruvate, coenzyme A (CoA), and oxidized nicotinamide adenine dinucleotide (NAD+) into acetyl-CoA, reduced form of nicotinamide adenine dinucleotide (NADH), and carbon dioxide. The activity of the PDC is up- and down-regulated by pyruvate dehydrogenase kinase and pyruvate dehydrogenase phosphatase, respectively. In addition, pyruvate is a key intermediate of glucose oxidation and an important precursor for the synthesis of glucose, glycerol, fatty acids, and nonessential amino acids.
The Sulwon Award for Scientific Achievement is the Korean Diabetes Association's highest scientific award and honors an individual who has excellently contributed to the progress in the field of diabetes and metabolism. Sulwon Award is named after an emeritus professor Eung Jin Kim, who founded Korean Diabetes Association. Prof. In-Kyu Lee received the fifth Sulwon Award at 2013 International Conference on Diabetes and Metabolism, November 6-9, 2013 at Seoul, Korea.
To maintain a continuous and steady supply of adenosine triphosphate (ATP) during the feed-fast cycle, cells must select fatty acid or glucose for fuel [1]. This process is largely controlled by the pyruvate dehydrogenase complex (PDC), which regulates the entry of glycolytic products into the tricarboxylic acid cycle by catalyzing the oxidative decarboxylation of pyruvate to acetyl-coenzyme A (CoA) in the mitochondria of mammalian cells [2]. The PDC is usually active during the fed-state in most tissues, where it suppresses pyruvate dehydrogenase kinase (PDK)-induced phosphorylation [3]. PDKs and pyruvate dehydrogenase phosphatases (PDPs) are key regulators of PDC activity, and they act in a phosphorylation-dephosphorylation manner [2]. The role of PDC in the regulation of glucose metabolism is briefly summarized in Fig. 1. In this review, I will discuss the correlation between PDC activity and metabolic diseases in humans.
The mammalian PDC is a large complex composed of three enzymes: pyruvate decarboxylase (E1 subunit), dihydrolipoyl acetyltransferase (E2 subunit), and dihydrolipoyl dehydrogenase (E3 subunit). The PDC catalyzes the oxidation of pyruvate to acetyl-CoA [4,5]. The PDC complex is inactivated by PDKs and activated by PDPs. Four PDK isoenzymes (PDK1, 2, 3, and 4) and two PDP isoenzymes (PDP1 and PDP2) are involved in this phosphorylation [6,7]. The four PDK isoenzymes are expressed in a tissue-specific manner [6]. PDK2 is highly expressed in heart, liver, and kidney of humans and rodents [8]. PDK4 is dominantly expressed in oxidative skeletal muscle, heart, lactating mammary gland, and liver [9,10]. PDK1 is expressed in heart [11] and pancreatic islets [12], while PDK3 expression has only been detected in testis, kidney, and brain [6].
The PDKs are transcriptionally regulated by insulin, glucocorticoids, thyroid hormone, and fatty acids. There is emerging evidence that transcriptional up-regulation of PDK [13,14,15] decreases PDC activity, which has been observed in several metabolic disorders, such as diabetes [16,17,18], heart disease [19,20], and fatty liver [21].
Metabolic inflexibility, defined as insufficient glucose utilization followed by increased lipolysis in the peripheral tissues, is a manifestation in patients with insulin resistance, obesity, and type 2 diabetes. A previous study has shown that PDK4 expression increases in the skeletal muscle of rats receiving a continuous infusion of intralipids (a fat emulsion), indicating a disruption in the suppression of PDK4 by insulin. These results also show a direct relationship between free fatty acid levels and PDK4 expression in the muscle [16]. Pdk4 levels are also elevated in fasting and diabetic individuals [9,22,23]. In contrast, high-fat fed, insulin-resistant mice lacking PDK4 exhibit lower blood glucose levels and better glucose tolerance than wild-type mice [24]. In mice that are null for the hepatic insulin receptor substrates 1 and 2, which is a novel model for type 2 diabetes, additional knockout of the PDK4 gene improved glycemic control and glucose tolerance [25].
In contrast, growth hormone (GH), whose function is opposite to that of insulin, stimulates PDK4 expression in the liver of wild-type mice during fasting by activating the janus kinase/signal transducer and activator of transcription (STAT5) pathway and increasing gluconeogenesis. Metformin inhibits GH-induced PDK4 expression via the AMP-activated protein kinase/small heterodimer partner-dependent pathway that inhibits the combination of STAT5 to the PDK4 promoter [26]. Additionally, overproduction of GH can increase the blood glucose level in patients with acromegaly. PDK2/PDK4 double-knockout mice are unable to tolerate long-term fasting (48 hours), succumbing to hypoglycemia, ketoacidosis, and hypothermia. These findings indicate that partial activation of the PDC, which inhibits PDK activity, may alleviate some symptoms of type 2 diabetes; however, complete activation of the PDC by inhibition of phosphorylation may be harmful and even fatal due to hypoglycemia and hypothermia [27,28].
Hepatic steatosis is closely associated with multiple metabolic abnormalities including increased fatty acid influx from the adipose tissue and de novo lipogenesis, decreased fatty acid oxidation and ketogenesis, and abnormal triacylglycerol secretion [29]. Previous study has shown that PDK4 expression is higher in the muscle than in the liver of insulin-resistant mice [24]. Thus, PDC in the liver is less active compared to that in the muscle of PDK4 knockout mice fed with high-fat diet (HFD), indicating that PDK4 plays a more important role in the muscle than in the liver [24]. Unpublished data from our laboratory has also shown the increase in PDK2 expression and the decrease in PDK4 expression in the liver of HFD-induced obese mice, demonstrating that PDK2 is primarily responsible for the inactivation of hepatic PDC activity during HFD feeding.
PDK4 overexpression in the heart of transgenic mice shows decreased glycolysis, increased fatty acid oxidation with metabolic inflexibility, and exacerbated cardiomyopathy. The mechanism of hypertrophy and fibrosis in cardiomyocytes of PDK4 Tg mice is associated with an increase in calcineurin expression, which is mediated by PDK4 [20]. By contrast, dichloroacetate (DCA), a PDK inhibitor, and PDK2/PDK4 deficiency show protective effects in the heart after ischemia [30]. DCA also has beneficial effects on right ventricular hypertrophy and pulmonary hypertension by increasing carbohydrate metabolism, reactive oxygen species production, and apoptosis and by decreasing smooth muscle cell proliferation in the right ventricle and pulmonary vasculature [31].
Recently, we found that PDK4 plays an important role in vascular calcification. Our unpublished data shows that PDK4 levels are up-regulated and the PDC was phosphorylated in cultured vascular smooth muscle cells and calcified vessels of patients with atherosclerosis. PDK4 promoted osteogenic differentiation of vascular smooth muscle cells by phosphorylating SMAD1/5/8 and enhancing bone morphogenic protein 2 signaling.
PDK expression is elevated in patients with diabetes, vascular calcification, heart failure, pulmonary hypertension, cancer and a variety of pathological conditions summarized in Table 1 [16,19,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45] and a disruption of PDK expression or the development of PDK inhibitors may help to treat these disorders. Although the mechanisms behind these beneficial effects are not entirely understood, PDC activity is critical in glucose utilization. The level of PDK4 is elevated in patients with type 2 diabetes and in animals and humans on a HFD. An increase in PDC activity by a PDK inhibitor enhances insulin activity by promoting glucose oxidation and lowering the blood glucose concentration. Therefore, small molecule inhibitors for PDKs are promising therapeutic agents for patients with metabolic syndrome.
Figures and Tables
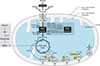 | Fig. 1Schematic representation of the regulation of glucose metabolism by pyruvate dehydrogenase complex (PDC). The activity of PDC is strongly inhibited by phosphorylation of its dehydrogenase component by pyruvate dehydrogenase kinases (PDKs) and enhanced by dephosphorylation by pyruvate dehydrogenase phosphatases (PDPs). The main regulatory factors of PDKs and PDPs are shown as above. Pyruvate enters into mitochondria via the voltage-dependent anion channel (VDAC) and mitochondrial pyruvate carrier (MPC) and is then converted into either oxaloacetate by pyruvate carboxylase or acetyl-CoA by PDC. Acetyl-CoA then enters into the tricarboxylic acid cycle, yielding nicotinamide adenine dinucleotide (NADH) and favin adenine dinucleotide 2 (FADH2) and promoting oxidative phosphorylation. PEP, phosphoenolpyruvate; CoASH, coenzyme A-SH; PEPCK, phosphoenolpyruvate carboxykinase; cAMP, cyclic adenosine monophosphate; ADP, adenosine diphosphate; ATP, adenosine triphosphate. |
ACKNOWLEDGMENTS
This work was supported the Korea Health technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant number: A111345).
References
1. Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev. 1998; 14:263–283.
2. Jeong JY, Jeoung NH, Park KG, Lee IK. Transcriptional regulation of pyruvate dehydrogenase kinase. Diabetes Metab J. 2012; 36:328–335.
3. Zhang S, Hulver MW, McMillan RP, Cline MA, Gilbert ER. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr Metab (Lond). 2014; 11:10.
4. Smolle M, Prior AE, Brown AE, Cooper A, Byron O, Lindsay JG. A new level of architectural complexity in the human pyruvate dehydrogenase complex. J Biol Chem. 2006; 281:19772–19780.
5. Sanderson SJ, Miller C, Lindsay JG. Stoichiometry, organisation and catalytic function of protein X of the pyruvate dehydrogenase complex from bovine heart. Eur J Biochem. 1996; 236:68–77.
6. Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998; 329(Pt 1):191–196.
7. Huang B, Gudi R, Wu P, Harris RA, Hamilton J, Popov KM. Isoenzymes of pyruvate dehydrogenase phosphatase. DNA-derived amino acid sequences, expression, and regulation. J Biol Chem. 1998; 273:17680–17688.
8. Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans. 2003; 31(Pt 6):1143–1151.
9. Wu P, Sato J, Zhao Y, Jaskiewicz J, Popov KM, Harris RA. Starvation and diabetes increase the amount of pyruvate dehydrogenase kinase isoenzyme 4 in rat heart. Biochem J. 1998; 329(Pt 1):197–201.
10. Wu P, Inskeep K, Bowker-Kinley MM, Popov KM, Harris RA. Mechanism responsible for inactivation of skeletal muscle pyruvate dehydrogenase complex in starvation and diabetes. Diabetes. 1999; 48:1593–1599.
11. Di RM, Feng QT, Chang Z, Luan Q, Zhang YY, Huang J, Li XL, Yang ZZ. PDK1 plays a critical role in regulating cardiac function in mice and human. Chin Med J (Engl). 2010; 123:2358–2363.
12. Sugden MC, Bulmer K, Augustine D, Holness MJ. Selective modification of pyruvate dehydrogenase kinase isoform expression in rat pancreatic islets elicited by starvation and activation of peroxisome proliferator-activated receptor-alpha: implications for glucose-stimulated insulin secretion. Diabetes. 2001; 50:2729–2736.
13. Attia RR, Sharma P, Janssen RC, Friedman JE, Deng X, Lee JS, Elam MB, Cook GA, Park EA. Regulation of pyruvate dehydrogenase kinase 4 (PDK4) by CCAAT/enhancer-binding protein beta (C/EBPbeta). J Biol Chem. 2011; 286:23799–23807.
14. Huang B, Wu P, Bowker-Kinley MM, Harris RA. Regulation of pyruvate dehydrogenase kinase expression by peroxisome proliferator-activated receptor-alpha ligands, glucocorticoids, and insulin. Diabetes. 2002; 51:276–283.
15. Kwon HS, Huang B, Unterman TG, Harris RA. Protein kinase B-alpha inhibits human pyruvate dehydrogenase kinase-4 gene induction by dexamethasone through inactivation of FOXO transcription factors. Diabetes. 2004; 53:899–910.
16. Kim YI, Lee FN, Choi WS, Lee S, Youn JH. Insulin regulation of skeletal muscle PDK4 mRNA expression is impaired in acute insulin-resistant states. Diabetes. 2006; 55:2311–2317.
17. Pehleman TL, Peters SJ, Heigenhauser GJ, Spriet LL. Enzymatic regulation of glucose disposal in human skeletal muscle after a high-fat, low-carbohydrate diet. J Appl Physiol (1985). 2005; 98:100–107.
18. Peters SJ, Harris RA, Wu P, Pehleman TL, Heigenhauser GJ, Spriet LL. Human skeletal muscle PDH kinase activity and isoform expression during a 3-day high-fat/low-carbohydrate diet. Am J Physiol Endocrinol Metab. 2001; 281:E1151–E1158.
19. Kong SW, Bodyak N, Yue P, Liu Z, Brown J, Izumo S, Kang PM. Genetic expression profiles during physiological and pathological cardiac hypertrophy and heart failure in rats. Physiol Genomics. 2005; 21:34–42.
20. Zhao G, Jeoung NH, Burgess SC, Rosaaen-Stowe KA, Inagaki T, Latif S, Shelton JM, McAnally J, Bassel-Duby R, Harris RA, Richardson JA, Kliewer SA. Overexpression of pyruvate dehydrogenase kinase 4 in heart perturbs metabolism and exacerbates calcineurin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008; 294:H936–H943.
21. Hwang B, Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) deficiency attenuates the long-term negative effects of a high-saturated fat diet. Biochem J. 2009; 423:243–252.
22. Wu P, Blair PV, Sato J, Jaskiewicz J, Popov KM, Harris RA. Starvation increases the amount of pyruvate dehydrogenase kinase in several mammalian tissues. Arch Biochem Biophys. 2000; 381:1–7.
23. Sugden MC, Holness MJ. Therapeutic potential of the mammalian pyruvate dehydrogenase kinases in the prevention of hyperglycaemia. Curr Drug Targets Immune Endocr Metabol Disord. 2002; 2:151–165.
24. Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008; 295:E46–E54.
25. Tao R, Xiong X, Harris RA, White MF, Dong XC. Genetic inactivation of pyruvate dehydrogenase kinases improves hepatic insulin resistance induced diabetes. PLoS One. 2013; 8:e71997.
26. Kim YD, Kim YH, Tadi S, Yu JH, Yim YH, Jeoung NH, Shong M, Hennighausen L, Harris RA, Lee IK, Lee CH, Choi HS. Metformin inhibits growth hormone-mediated hepatic PDK4 gene expression through induction of orphan nuclear receptor small heterodimer partner. Diabetes. 2012; 61:2484–2494.
27. Jeoung NH, Rahimi Y, Wu P, Lee WN, Harris RA. Fasting induces ketoacidosis and hypothermia in PDHK2/PDHK4-double-knockout mice. Biochem J. 2012; 443:829–839.
28. Jeoung NH, Harris CR, Harris RA. Regulation of pyruvate metabolism in metabolic-related diseases. Rev Endocr Metab Disord. 2014; 15:99–110.
29. Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004; 114:147–152.
30. Ussher JR, Wang W, Gandhi M, Keung W, Samokhvalov V, Oka T, Wagg CS, Jaswal JS, Harris RA, Clanachan AS, Dyck JR, Lopaschuk GD. Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovasc Res. 2012; 94:359–369.
31. Piao L, Sidhu VK, Fang YH, Ryan JJ, Parikh KS, Hong Z, Toth PT, Morrow E, Kutty S, Lopaschuk GD, Archer SL. FOXO1-mediated upregulation of pyruvate dehydrogenase kinase-4 (PDK4) decreases glucose oxidation and impairs right ventricular function in pulmonary hypertension: therapeutic benefits of dichloroacetate. J Mol Med (Berl). 2013; 91:333–346.
32. Velpula KK, Bhasin A, Asuthkar S, Tsung AJ. Combined targeting of PDK1 and EGFR triggers regression of glioblastoma by reversing the Warburg effect. Cancer Res. 2013; 73:7277–7289.
33. Nakai N, Obayashi M, Nagasaki M, Sato Y, Fujitsuka N, Yoshimura A, Miyazaki Y, Sugiyama S, Shimomura Y. The abundance of mRNAs for pyruvate dehydrogenase kinase isoenzymes in brain regions of young and aged rats. Life Sci. 2000; 68:497–503.
34. Kulkarni SS, Salehzadeh F, Fritz T, Zierath JR, Krook A, Osler ME. Mitochondrial regulators of fatty acid metabolism reflect metabolic dysfunction in type 2 diabetes mellitus. Metabolism. 2012; 61:175–185.
35. Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D, Abdulkarim B, McMurtry MS, Petruk KC. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010; 2:31ra4.
36. Sameen S, Khalid Z, Malik SI. Role of pyruvate dehydrogenase kinases (PDK's) and their respective microRNA's in human ovarian cancer. J Med Genet Genomics. 2011; 3:115–121.
37. Kennerson ML, Yiu EM, Chuang DT, Kidambi A, Tso SC, Ly C, Chaudhry R, Drew AP, Rance G, Delatycki MB, Zuchner S, Ryan MM, Nicholson GA. A new locus for X-linked dominant Charcot-Marie-Tooth disease (CMTX6) is caused by mutations in the pyruvate dehydrogenase kinase isoenzyme 3 (PDK3) gene. Hum Mol Genet. 2013; 22:1404–1416.
38. Nellemann B, Vendelbo MH, Nielsen TS, Bak AM, Hogild M, Pedersen SB, Bienso RS, Pilegaard H, Moller N, Jessen N, Jorgensen JO. Growth hormone-induced insulin resistance in human subjects involves reduced pyruvate dehydrogenase activity. Acta Physiol (Oxf). 2013; 10. 21. Epub. DOI: 10.1111/apha.12183.
39. Huang J, Jones D, Luo B, Sanderson M, Soto J, Abel ED, Cooksey RC, McClain DA. Iron overload and diabetes risk: a shift from glucose to fatty acid oxidation and increased hepatic glucose production in a mouse model of hereditary hemochromatosis. Diabetes. 2011; 60:80–87.
40. Connaughton S, Chowdhury F, Attia RR, Song S, Zhang Y, Elam MB, Cook GA, Park EA. Regulation of pyruvate dehydrogenase kinase isoform 4 (PDK4) gene expression by glucocorticoids and insulin. Mol Cell Endocrinol. 2010; 315:159–167.
41. Arikawa E, Ma RC, Isshiki K, Luptak I, He Z, Yasuda Y, Maeno Y, Patti ME, Weir GC, Harris RA, Zammit VA, Tian R, King GL. Effects of insulin replacements, inhibitors of angiotensin, and PKCbeta's actions to normalize cardiac gene expression and fuel metabolism in diabetic rats. Diabetes. 2007; 56:1410–1420.
42. Mori J, Basu R, McLean BA, Das SK, Zhang L, Patel VB, Wagg CS, Kassiri Z, Lopaschuk GD, Oudit GY. Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Circ Heart Fail. 2012; 5:493–503.
43. Mallinson JE, Constantin-Teodosiu D, Glaves PD, Martin EA, Davies WJ, Westwood FR, Sidaway JE, Greenhaff PL. Pharmacological activation of the pyruvate dehydrogenase complex reduces statin-mediated upregulation of FOXO gene targets and protects against statin myopathy in rodents. J Physiol. 2012; 590:6389–6402.
44. Wang Y, Liu W, Masuyama R, Fukuyama R, Ito M, Zhang Q, Komori H, Murakami T, Moriishi T, Miyazaki T, Kitazawa R, Yoshida CA, Kawai Y, Izumi S, Komori T. Pyruvate dehydrogenase kinase 4 induces bone loss at unloading by promoting osteoclastogenesis. Bone. 2012; 50:409–419.
45. Kamarajugadda S, Stemboroski L, Cai Q, Simpson NE, Nayak S, Tan M, Lu J. Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol. 2012; 32:1893–1907.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download