Abstract
Background
Methods
Results
Figures and Tables
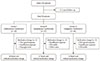 | Fig. 1Flow of the study. A total of 116 patients were enrolled, and 99 subjects were analyzed in the study. Of these patients, 28 in the glimepiride/metformin group, 27 in the pioglitazone/metformin group, and 33 in the sitagliptin/metformin group completed the study without medication change. GI, gastrointestinal. |
 | Fig. 2Change in hemoglobin A1c (HbA1c) from baseline to 24 weeks. Solid lines indicate the median HbA1c level, and broken lines indicate the median of individually assessed differences in HbA1c level from baseline. Multiple linear regression was used for statistical analysis, and group I was used as a reference group. The change of HbA1c was not statistically different among groups at 12 weeks (group II, P=0.101; group III, P=0.673) or 24 weeks (group II, P=0.066; group III, P=0.678) after adjustment for baseline age, sex, body mass index, and baseline HbA1c level. |
 | Fig. 3(A) The proportions of individuals who achieved hemoglobin A1c (HbA1c) level ≤7% at 24 weeks, and (B) the proportions of individuals with overall successful diabetes control according to medication. (A) Dark gray bar indicates the proportion of individuals who achieved an HbA1c level ≤7% among those who did not change medications by the end of the study. Fisher exact test was used to compare the three groups, and there was no significant difference (P=0.649). (B) Light gray bar indicates the proportion of individuals, among the total number of subjects who initially enrolled in the study, except for those who did not continue with follow-up observation, who achieved an HbA1c level ≤7% at 24 weeks or reduced medication during the study period because of a very good response. The Fisher exact test was used to compare the three groups, and there was no significant difference (P=0.593). |
 | Fig. 4Change in hemoglobin A1c (HbA1c) according to baseline HbA1c level. Dark gray bar indicates the level at baseline and light gray bar at 24 weeks. Bars represent medians (low quartile, high quartile). Despite different initial HbA1c levels (8.2% vs. 9.9% vs. 11.9%; P<0.001), HbA1c level in each category after 24 weeks of treatment demonstrated no statistical difference by Kruskal-Wallis test (6.4% vs. 6.6% vs. 6.0%; P=0.051). |
 | Fig. 5(A) The proportions of individuals who achieved an hemoglobin A1c (HbA1c) level ≤7% at 24 weeks, and (B) the proportions of individuals with overall successful diabetes control according to baseline HbA1c level. Category I, 7.5%≤HbA1c<9.0; category II, 9.0%≤HbA1c<11.0; category III, 11.0%≤ HbA1c. (A) Dark gray bar indicates the proportion of individuals who achieved an HbA1c level ≤7% among those who did not change medications by the end of the study. Fisher exact test was used to compare the three groups, and there was no significant difference (P=1.000). (B) Light gray bar indicates the proportion of individuals, among the total number subjects who initially enrolled in the study, except for those who did not continue with follow-up observation, who achieved an HbA1c level ≤7% at 24 weeks or reduced medication during the study period because of a very good response. Fisher exact test was used to compare the three groups, and there was no significant difference (P=0.819). |
Table 1

Values are presented as mean±standard deviation, number (%), or median (low quartile, high quartile). Analysis of variance test was used for parametric analysis and Kruskal-Wallis test for nonparametric analysis.
BMI, body mass index; FBS, fasting blood glucose; PPG, postprandial glucose; HbA1c, hemoglobin A1c; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; HOMA-β, homeostasis model assessment of β-cell function; HOMA-IR, homeostasis model assessment of insulin resistance; BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Table 2

In model 1, which adjusts for baseline age, sex, BMI, and HbA1c, there was no significant difference between groups in change of HbA1c (group II, P=0.066; group III, P=0.678). Additional adjustment of HOMA-β and HOMA-IR did not alter the statistical significance. However, after additional adjustment of daily metformin dose, group II showed a statistically smaller reduction in HbA1c than group I (covariate-adjusted difference in change of HbA1c, 0.35%; P=0.046).
HbA1c, hemoglobin A1c; BMI, body mass index; HOMA-β, homeostasis model assessment of β-cell function; HOMA-IR, homeostasis model assessment of insulin resistance.
Table 3

Values are presented as median (low quartile, high quartile), mean±standard deviation, or number (%). Analysis of variance test was used for parametric analysis and Kruskal-Wallis test for nonparametric analysis.
BMI, body mass index; HbA1c, hemoglobin A1c; HOMA-β, homeostasis model assessment of β-cell function; HOMA-IR, homeostasis model assessment of insulin resistance.
aStatistical significance (P<0.017) in post hoc analysis (Mann-Whitney test with Bonferroni correction) was indicated by category I vs. III, bCategory II vs. III, cCategory I vs. II.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download