Abstract
Brown adipose tissue (BAT) is recognized as the major site of sympathetically activated nonshivering thermogenesis during cold exposure and after spontaneous hyperphagia, thereby controling whole-body energy expenditure and body fat. In adult humans, BAT has long been believed to be absent or negligible, but recent studies using fluorodeoxyglucose-positron emission tomography, in combination with computed tomography, demonstrated the existence of metabolically active BAT in healthy adult humans. Human BAT is activated by acute cold exposure, being positively correlated to cold-induced increases in energy expenditure. The metabolic activity of BAT differs among individuals, being lower in older and obese individuals. Thus, BAT is recognized as a regulator of whole-body energy expenditure and body fat in humans as in small rodents, and a hopeful target combating obesity and related disorders. In fact, there are some food ingredients such as capsaicin and capsinoids, which have potential to activate and recruit BAT via activity on the specific receptor, transient receptor potential channels, thereby increasing energy expenditure and decreasing body fat modestly and consistently.
We are now facing a worldwide epidemic of obesity, a major risk factor in the development of common diseases such as diabetes mellitus, dyslipidemias, fatty liver, hypertension, and arteriosclerosis. Obesity is the state of excessive accumulation of triglyceride in adipose tissues because of a prolonged positive energy balance. In mammals, there are two types of adipose tissue, white and brown adipose tissues (BAT). The two tissues are similar in their major population of adipocyte having intracellular lipid droplets, but are quite different in the physiological functions. White adipose tissue (WAT) is the primary site of energy storage, while BAT is specialized for energy expenditure. In small rodents, BAT is recognized as the major site of sympathetically activated nonshivering thermogenesis during cold exposure and after spontaneous hyperphagia, thereby controling whole-body energy expenditure and body fat [1,2]. In adult humans, BAT has long been believed to be absent or negligible, but recent studies using fluorodeoxyglucose (FDG)-positron emission tomography (PET), in combination with computed tomography (CT), demonstrated the existence of metabolically active BAT in adult humans [3]. This review summarizes recent knowledge on human BAT, with special reference to its thermogenic and antiobesity activities.
The structure of adipocytes in WAT is characterized by a single large lipid droplet and a small number of mitochondria, while adipocytes in BAT contain numerous small lipid droplets surrounded by a large number of mitochondria. In BAT mitochondria, there is a unique uncoupling protein 1 (UCP1) in the inner membrane [4]. As UCP1 is expressed selectively in BAT, but not in other tissues including WAT, it is recognized as a specific molecular marker of brown adipocyte. UCP1 acts to uncouple oxidative phosphorylation from ATP synthesis, thereby dissipating energy as heat. BAT thermogenesis is directly regulated by sympathetic nerves distributed abundantly to this tissue (Fig. 1). The cellular events associated with sympathetic activation of BAT thermogenesis are the binding of norepinephrine released from sympathetic nerve terminals to b-adrenergic receptors, the activation of adenylate cyclase and hormone-sensitive lipase, which hydrolyzes intracellular triglyceride. The released fatty acids activate UCP1 and are oxidized in mitochondria to serve as an energy source of thermogenesis.
Thus, the principal substrate for BAT thermogenesis is fatty acids from triglyceride in this tissue and also from circulating free fatty acids and lipoproteins. Sympathetic activation also results in an increased fat mobilization in WAT, and released fatty acids are used in BAT as well as muscle tissues. Together with fatty acids, glucose is also an important fuel in BAT, probably not as a direct substrate for thermogenesis but as a carbon source for fatty acid synthesis and rapid oxidation of fatty acids. Moreover, as UCP1, because of its uncoupling activity, decreases the cellular energy charge, glucose is indispensable for rapid recovery of cellular ATP levels by activation of anaerobic glycolysis [5,6].
Most information about BAT has come from experimental studies in small rodents. In humans, significant amounts of BAT are present in newborns and may contribute to body temperature regulation during the neonatal period, probably in the same way as in small rodents. However, BAT seems to disappear rapidly during postnatal periods and in adults is rather difficult to identify by conventional anatomical and histological examinations [7]. Thus, it has been a general contention that BAT is absent or of minute amounts and plays negligible, if any, roles in adult humans [8,9]. The existence of metabolically active BAT in adult humans has been suggested by the clinical studies using FDG-PET, one of the powerful diagnostic tools for malignant tumors: that is, PET sometimes detects symmetrical FDG uptake in the shoulder and thoracic spine regions, where no tumor is present. By simultaneous examinations with PET and X-ray CT, the site of the FDG uptake was identified as adipose tissue, named as "USA-fat" [10]. Such FDG uptake is increased at lower environmental temperatures [10,11], and reduced by pretreatment with b-adrenergic blockers [12,13]. Animal studies demonstrated that β-adrenergically stimulated 2-deoxyglucose uptake into BAT is totally dependent on the activation of UCP1 [6]. These findings collectively suggest that the FDG uptake in adipose tissue at the specific regions reflects the metabolic activity of BAT.
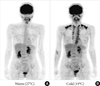
The presence of BAT in adult humans was confirmed by the experimental FDG-PET/CT studies for healthy volunteers [14-17]. For example, when exposed to cold (19℃) with light clothing for 2 hours, some participants show a substantial FDG uptake into adipose tissue in the supraclavicular and paraspinal regions (Fig. 2), whereas they showed no detectable uptake when kept warm (27℃). Histological examinations revealed the presence of UCP-1 positive brown adipocytes in these regions. Our study for 213 healthy participants aged 20 to 73 years revealed that the prevalence of cold-activated BAT was more than 50% in young subjects of the twenties, decreased with age, and in less than 10% of the fifties and sixties [18]. It is to be noted that BAT can be well identified by FDG-PET/CT only when exposed to acute cold in winter [15]. This may be a reason of the low (<10%) prevalence of BAT in clinical studies [19-23], in which FDG-PET/CT is carried out at warm conditions at around 24℃ with normal clothing.
It has been established in small rodents that BAT is the major site for adaptive nonshievering thermogenesis, a physiological process during which heat production increases in response to environmental changes. One of the typical examples is cold-induced thermogenesis. When mammals are exposed to cold, they maintain body temperature autonomically not only by decreasing heat loss from body surface but also by increasing heat production. At least two mechanisms contribute to cold-induced thermogenesis, shivering of skeletal muscle and activation of BAT. The critical role of BAT for cold-induced thermogenesis was clearly confirmed by a finding that mice lacking UCP1 is unable to maintain body temperature under a cold environment and die in hours [24].
In humans, the fact that the metabolic activity of BAT is increased after cold exposure suggests a contribution of BAT to cold-induced thermogenesis. In fact, we [25] and Orava et al. [26], by measuring whole-body energy expenditure in participants showing detectable cold-activated FDG uptake (BAT-positive group) and also those showing undetectable uptake (BAT-negative group), demonstrated that energy expenditure after acute cold exposure was higher in the BAT-positive than -negative group, while the basal energy expenditure at warm conditions was almost comparable in the two groups. A positive correlation was found between the cold-induced rise in energy expenditure and the BAT activity quantified from FDG uptake [25]. These findings clearly indicate a significant role of BAT for cold-induced thermogenesis in humans. Moreover, we [25] found that the response of the skin temperature to cold exposure was different between the BAT-positive and -negative groups; that is, the cold-induced temperature drop in the skin region close to BAT deposits is smaller in the BAT-positive group, while no significant difference in other skin regions apart from BAT deposits. These results indicate that BAT thermogenesis is actually activated during cold exposure and probably contributes to body temperature regulation.
Another example of adaptive thermogenesis is diet-induced thermogenesis, an increase in energy expenditure seen after food intake, which is sometimes called as "postprandial thermogenesis" or "thermic effect of food." Since the first report of BAT hyperplasia in small rodents kept on prolonged overfeeding on highly palatable cafeteria-diets [27], BAT has been proposed as a site of diet-induced thermogenesis [1]. It is well known that a change in energy intake results in an adaptive change in energy expenditure so that body energy content is kept at steady levels. In support of this, it has repeatedly been reported that BAT mass and/or UCP1 expression are decreased in animals deprived from food but increased in those fed on high fat diets [2]. However, it is to be noted that the effects of chronic hyperphagia may not be equal to the cumulative effects of single ingestions of food. To our knowledge, there has been no report showing direct evidence for the involvement of BAT in diet-induced thermogenesis in small rodents [28]. In humans, however, BAT may contribute, at least in part, to diet-induced thermogenesis, because whole-body energy expenditure increased after an oral ingestion of food more in BAT-positive than -negative participants, particularly during the initial period of 1 hour (Aita et al., unpublished observations).
Thus, it seems likely that human BAT is involved in adaptive thermogenesis, thereby regulating whole-body energy expenditure. This is supported by some previous studies examining the effects of UCP1 gene polymorphism. In the human UCP1 gene, there is a single nucleotide substitution at -3826A to G [29], which lowers UCP1 mRNA expression [30,31], accelerates the age-related reduction of BAT prevalence [18,32], and attenuates cold- and diet-induced thermogenesis [33,34].
Consistent with the significant role of BAT in short-term regulation of energy expenditure, there have been piles of evidence for BAT as a long-term regulator of energy balance and body fat content: that is, for examples, almost all obese model animals express lower levels of UCP1 in BAT, while mice over-expressing UCP1 are leaner, and prolonged cold exposure and/or b3-adrenoceptor agonist treatment result in a reduction of body fat associated with BAT hyperplasia [1,2]. Enerback et al. [24] first reported that mice lacking UCP1 are cold-sensitive but not obese. This paradoxical finding was re-examined by Kontani et al. [35], who demonstrated that these mice get obese when they are kept on a high-fat diet for more than half a year. Feldmann et al. [36] also reported that mice lacking UCP1 are susceptible to diet-induced obesity when they are maintained at thermoneutral temperatures (~29℃), but not at conventional animal room temperatures (18℃ to 22℃). Thus, the preventive effect of UCP1 against obesity is actualized only when animals need not to increase cold-induced thermogenesis, indicating some interactive effects of cold and food on BAT and whole-body energy expenditure.
In humans, both clinical and experimental studies have consistently shown significant inverse relationships between the activity/prevalence of BAT and adiposity-related parameters such as body mass index (BMI), body fat content, and visceral fat accumulation. Retrospective readings of FDG-PET/CT in thousands of patients have revealed that BAT prevalence is lower in patients with higher BMI [19,21,22]. Prospective studies in healthy participants also demonstrated that the prevalence and activity of cold-activated BAT deceased with increasing adiposity [15,16]. The apparent association between BAT prevalence and adiposity, however, is to be carefully evaluated, because these are considerably influenced by age. In fact, the mean age is lower in the BAT-positive participant group than the BAT-negative group. More detailed analysis revealed that the prevalence of cold-activated BAT is more than 50% in young subjects of the twenties, decreased with age, and in less than 10% of the fifties and sixties [18]. A strong impact of age on BAT prevalence has also been reported in various clinical studies [19,21,22]. On the other hand, it is well known that the aging process produces notable changes in body composition: that is, in general, percent body fat increases while lean mass and bone mineral density decreases [37]. Thus, it seems likely that age-related accumulation of body fat is associated with decreased BAT activity (Fig. 3). This is supported by the findings that the adiposity-related parameters increased with age in the BAT-negative group, while they remained unchanged from the twenties to forties in the BAT-positive group [18].
The finding that BAT is protective against body fat accumulation has encouraged the search how to activate or recruit BAT (Fig. 3). This is particularly intriguing because people with lower or undetectable BAT activities are more obese and to be treated. As noted previously, cold seems the most physiological and powerful stimulus for activation of BAT. Mice exposed to cold at 4℃, for example, show a substantial increase in sympathetic nerve activity in BAT, followed by the serial activation of the intracellular events shown in Fig. 1. Furthermore, prolonged cold exposure elicits an increased UCP1 expression, mitochondriogenesis, and proliferation and differentiation of brown adipocyte, all of which result in BAT hyperplasia and an increased thermogenic capacity [1]. Particularly interesting is that chronic sympathetic activation produces not only BAT hyperplasia but also a remarkable induction of UCP1-positive brown-like adipocytes in white fat pads, called as "beige or brite" cells [38-40]. These beige cells belong to a cell lineage different from "classical" brown adipocytes, but also contribute to adaptive thermogenesis and body fat reduction [41-43]. Very recently, Wu et al. [44] identified some genes expressed selectively in mouse beige cells and found their high levels of expression in human supraclavicular fat deposits identified as BAT by FDG-PET/CT. Lee et al. [45] reported that preadipocytes isolated from human supravicular fat were capable of differentiating into UCP1-positive adipocytes in vitro, regardless of FDG-PET status. Moreover, we found that BAT activity in humans is remarkably increased during winter in individuals who showed undetectable activities in summer [22]. All these data suggest that human BAT identified by FDG-PET/CT is largely composed of beige cells and is inducible in response to appropriate sympathetic stimulation. In fact, when our participants with undetectable or low BAT activity were kept in a cold environment at 15℃ to 17℃ for 2 hours every day for 6 weeks, their BAT activity was significantly increased (Yoneshiro et al., unpublished data). More importantly, the change in BAT activity was negatively correlated with those in body fat content. These results indicate that human BAT can be induced and/or recruited, and is involved in reducing body fat.
Although daily cold exposure can recruit human BAT, it would seem difficult to increase exposure to cold in daily life. It is now well established that cold stimulus is received by transient receptor potential channels (TRP) (Fig. 1). Among the members of the TRP family, TRPM8 and TRPA1 are the most likely receptor candidates sensitive to low temperatures [46]. The mean activation temperatures of TRPA1 and TRPM8 are around 20℃, being comparable with those applied in human studies to activate BAT. Accordingly, chemical activation of these receptors would mimic the effects of cold exposure. Actually, there are various ingredients in food acting as agonists for these TRPs [46], a representative of which is menthol, a cooling and flavor compound in mint, acting on TRPM8. TRPA1 is activated by allyl- and benzyl-isothiocyanates, pungent elements in mustard and Wasabi (Japanese horseradish). Among the TRP agonists, the most extensively studied is capsaicin, a pungent principle of chili pepper, which is a potent agonist for TRPV1. There have been many animal studies demonstrating that capsaicin and its nonpungent analogs (capsinoids) increase BAT thermogenesis through the activation of TRPV1 and the sympathetic nervous system, and decrease body fat. Recent human studies have also confirmed similar thermogenic and antiobesity effects of capsinoids [47-49]. Thus, capsaicin/capsinoids as well as other food ingredients activating TRPs are promising as an antiobesity regimen easily applicable in daily life.
In addition to the regulatory roles of BAT in energy expenditure and body fat content, BAT may be relevant to some metabolic disorders. It has repeatedly been reported that cold acclimated animals show improved insulin sensitivity and enhanced glucose utilization in various peripheral tissues including BAT [50-53]. Bartelt et al. [54] visualized the in vivo processing of lipoproteins and demonstrated a regulatory role of BAT in plasma triglyceride metabolism in hyperlipidemia and obesity. Moreover, Nishio et al. [55] reported augmented glucose and lipid tolerance in mice transplanted with functional brown adipocytes developed from human pluripotent stem cells. These findings, together with a report that the prevalence and activity of BAT is decreased in diabetic patients [22], suggest that BAT may be involved in the etiology of diabetes mellitus and dyslipidemias, independently of and/or secondly to obesity. Thus, BAT may be an intriguing target for combating not only obesity but also some related diseases.
Figures and Tables
Fig. 1
Sympathetically activated thermogenesis in brown adipose tissue, lipid mobilization from white adipose tissue, and induction of beige cells. Sympathetic nerve activity in adipose tissues is increased in response to cold exposure and oral ingestion of some food ingredients through the activation of transient receptor potential channels (TRP). Noradrenaline binds to β-adrenergic receptors (βAR) and initiates signaling cascades for triglyceride (TG) hydrolysis. The released fatty acids activate uncoupling protein 1 (UCP1) and are oxidized to serve as an energy source of thermogenesis. Activated UCP1 uncouples oxidative phosphorylation from ATP synthesis and dissipates energy as heat. Chronic sympathetic activation produces not only brown fat hyperplasia but also an induction of beige cells in white fat, thereby increasing whole-body energy expenditure and decreasing body fat.
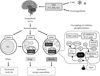
Fig. 2
Human brown adipose tissue detected by fluorodeoxyglucose (FDG)-positron emission tomography (PET). FDG uptake into adipose tissue at the supraclavicular and paraspinal regions is detected by PET. The FDG uptake into adipose tissues is negligible under a warm condition at 27℃ (A), but increases greatly after exposure to cold at 19℃ (B) for 2 hours.
ACKNOWLEDGMENTS
This work was supported by Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (22590227, 24240092), and a Special Research Grant from Tenshi College.
References
1. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004. 84:277–359.
2. Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000. 404:652–660.
3. Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007. 293:E444–E452.
4. Ricquier D, Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem J. 2000. 345(Pt 2):161–179.
5. Shimizu Y, Nikami H, Saito M. Sympathetic activation of glucose utilization in brown adipose tissue in rats. J Biochem. 1991. 110:688–692.
6. Inokuma K, Ogura-Okamatsu Y, Toda C, Kimura K, Yamashita H, Saito M. Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes. 2005. 54:1385–1391.
7. Himms-Hagen J. Does brown adipose tissue (BAT) have a role in the physiology or treatment of human obesity? Rev Endocr Metab Disord. 2001. 2:395–401.
8. Cinti S. The role of brown adipose tissue in human obesity. Nutr Metab Cardiovasc Dis. 2006. 16:569–574.
9. Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972. 112(Pt 1):35–39.
10. Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat ("USA-Fat"): description on 18F-FDG PET/CT. J Nucl Med. 2003. 44:170–176.
11. Garcia CA, Van Nostrand D, Atkins F, Acio E, Butler C, Esposito G, Kulkarni K, Majd M. Reduction of brown fat 2-deoxy-2-[F-18]fluoro-D-glucose uptake by controlling environmental temperature prior to positron emission tomography scan. Mol Imaging Biol. 2006. 8:24–29.
12. Parysow O, Mollerach AM, Jager V, Racioppi S, San Roman J, Gerbaudo VH. Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clin Nucl Med. 2007. 32:351–357.
13. Soderlund V, Larsson SA, Jacobsson H. Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. Eur J Nucl Med Mol Imaging. 2007. 34:1018–1022.
14. Saito M, Okamatsu-Ogura Y, Tsujisaki M, Kaji T, Nakada K. Human brown adipose tissue evaluated by FDG-PET: activation by cold exposure. Int J Obes (Lond). 2007. 31:Suppl 1. S32.
15. Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009. 58:1526–1531.
16. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009. 360:1500–1508.
17. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009. 360:1518–1525.
18. Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, Miyagawa M, Tsujisaki M, Saito M. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring). 2011. 19:1755–1760.
19. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009. 360:1509–1517.
20. Au-Yong IT, Thorn N, Ganatra R, Perkins AC, Symonds ME. Brown adipose tissue and seasonal variation in humans. Diabetes. 2009. 58:2583–2587.
21. Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, Reimold M, Haring HU, Claussen CD, Stefan N. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010. 59:1789–1793.
22. Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, Richard D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011. 96:192–199.
23. Nedergaard J, Bengtsson T, Cannon B. Three years with adult human brown adipose tissue. Ann N Y Acad Sci. 2010. 1212:E20–E36.
24. Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997. 387:90–94.
25. Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y, Saito M. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring). 2011. 19:13–16.
26. Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerback S, Virtanen KA. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011. 14:272–279.
27. Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979. 281:31–35.
28. Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010. 11:263–267.
29. Del Mar Gonzalez-Barroso M, Ricquier D, Cassard-Doulcier AM. The human uncoupling protein-1 gene (UCP1): present status and perspectives in obesity research. Obes Rev. 2000. 1:61–72.
30. Rose G, Crocco P, D'Aquila P, Montesanto A, Bellizzi D, Passarino G. Two variants located in the upstream enhancer region of human UCP1 gene affect gene expression and are correlated with human longevity. Exp Gerontol. 2011. 46:897–904.
31. Esterbauer H, Oberkofler H, Liu YM, Breban D, Hell E, Krempler F, Patsch W. Uncoupling protein-1 mRNA expression in obese human subjects: the role of sequence variations at the uncoupling protein-1 gene locus. J Lipid Res. 1998. 39:834–844.
32. Yoneshiro T, Ogawa T, Okamoto N, Matsushita M, Aita S, Kameya T, Kawai Y, Iwanaga T, Saito M. Impact of UCP1 and beta3AR gene polymorphisms on age-related changes in brown adipose tissue and adiposity in humans. Int J Obes (Lond). Epub 2012 Oct 2. DOI: http://dx.doi.org/10.1038/ijo.2012.161.
33. Nagai N, Sakane N, Fujishita A, Fujiwara R, Kimura T, Kotani K, Moritani T. The -3826 A-->G variant of the uncoupling protein-1 gene diminishes thermogenesis during acute cold exposure in healthy children. Obes Res Clin Pract. 2007. 1:99–107.
34. Nagai N, Sakane N, Ueno LM, Hamada T, Moritani T. The -3826 A-->G variant of the uncoupling protein-1 gene diminishes postprandial thermogenesis after a high fat meal in healthy boys. J Clin Endocrinol Metab. 2003. 88:5661–5667.
35. Kontani Y, Wang Y, Kimura K, Inokuma KI, Saito M, Suzuki-Miura T, Wang Z, Sato Y, Mori N, Yamashita H. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell. 2005. 4:147–155.
36. Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009. 9:203–209.
37. St-Onge MP, Gallagher D. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. 2010. 26:152–155.
38. Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992. 103(Pt 4):931–942.
39. Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998. 102:412–420.
40. Inokuma K, Okamatsu-Ogura Y, Omachi A, Matsushita Y, Kimura K, Yamashita H, Saito M. Indispensable role of mitochondrial UCP1 for antiobesity effect of beta3-adrenergic stimulation. Am J Physiol Endocrinol Metab. 2006. 290:E1014–E1021.
41. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010. 285:7153–7164.
42. Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010. 11:257–262.
43. Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011. 121:96–105.
44. Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012. 150:366–376.
45. Lee P, Swarbrick MM, Zhao JT, Ho KK. Inducible brown adipogenesis of supraclavicular fat in adult humans. Endocrinology. 2011. 152:3597–3602.
46. Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007. 292:R64–R76.
47. Whiting S, Derbyshire E, Tiwari BK. Capsaicinoids and capsinoids A potential role for weight management? A systematic review of the evidence. Appetite. 2012. 59:341–348.
48. Yoneshiro T, Aita S, Kawai Y, Iwanaga T, Saito M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am J Clin Nutr. 2012. 95:845–850.
49. Saito M, Yoneshiro T. Capsinoids and related food ingredients activating brown fat thermogenesis and reducing body fat in humans. Curr Opin Lipidol. 2013. 24:71–77.
50. Vallerand AL, Perusse F, Bukowiecki LJ. Cold exposure potentiates the effect of insulin on in vivo glucose uptake. Am J Physiol. 1987. 253(2 Pt 1):E179–E186.
51. Vallerand AL, Perusse F, Bukowiecki LJ. Stimulatory effects of cold exposure and cold acclimation on glucose uptake in rat peripheral tissues. Am J Physiol. 1990. 259(5 Pt 2):R1043–R1049.
52. Shimizu Y, Nikami H, Tsukazaki K, Machado UF, Yano H, Seino Y, Saito M. Increased expression of glucose transporter GLUT-4 in brown adipose tissue of fasted rats after cold exposure. Am J Physiol. 1993. 264(6 Pt 1):E890–E895.
53. Gasparetti AL, de Souza CT, Pereira-da-Silva M, Oliveira RL, Saad MJ, Carneiro EM, Velloso LA. Cold exposure induces tissue-specific modulation of the insulin-signalling pathway in Rattus norvegicus. J Physiol. 2003. 552(Pt 1):149–162.
54. Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmuller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011. 17:200–205.
55. Nishio M, Yoneshiro T, Nakahara M, Suzuki S, Saeki K, Hasegawa M, Kawai Y, Akutsu H, Umezawa A, Yasuda K, Tobe K, Yuo A, Kubota K, Saito M. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 2012. 16:394–406.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download