Abstract
Vision loss in diabetic retinopathy (DR) is attributable to retinal vascular disorders that result in macular edema and neoangiogenesis. In addition to laser photocoagulation therapy, intraocular injections of antivascular endothelial growth factor drugs have contributed to the treatment of these disease conditions. Nonetheless, the clinical feasibility of intraocular drug administration has raised an increasing demand to develop alternative drugs that can fundamentally ameliorate the retinal vascular dysfunctions in DR. For this purpose, experimental animal models that reproduce human DR would be of clinical benefit. Despite the unavailability of DR models in rats or mice, pharmacological and genetic manipulations without hyperglycemia have successfully recapitulated retinal edema and neoangiogenesis in postnatal mouse retinas, thereby enabling the understanding of the pathophysiology underlying DR. This article highlights the utility of experimental mouse models of retinal vascular abnormalities and discusses cellular and molecular mechanisms responsible for the onset and progression of DR. These approaches will lead to the identification of novel drug targets for the restoration of vascular integrity and regeneration of functional capillaries in DR.
Diabetic retinopathy (DR) is a leading cause of vision loss in working-age populations. In patients with diabetes, the overall prevalence of DR is approximately 35% [1]. While DR is clinically categorized into nonproliferative and proliferative types, diabetic macular edema (DME; present in 25% of persons with diabetes) affects central vision with metamorphopsia and micropsia in any type of DR [2]. In proliferative DR, obstruction of retinal capillaries leads to upregulation of proangiogenic growth factors, including vascular endothelial growth factor (VEGF), in hypoxic retinas [3]. Consequently, neoangiogenesis, an outgrowth of new blood vessels into the posterior surface of the vitreous, directly causes vision-threatening hemorrhage and retinal detachment [4]. While laser photocoagulation has been the standard modality to suppress DME and neoangiogenesis, intraocular injections of anti-VEGF drugs, either alone or in combination with surgical interventions, have globally become an additional option [5,6]. Although the intraocular anti-VEGF therapy has raised awareness of the feasibility of pharmacological treatment for DR, its limited efficacy has evoked the necessity to identify new drug targets for the fundamental treatment of DME and neoangiogenesis [7]. While diabetes animal models fail to reproduce human DR pathophysiology, technical advances in molecular manipulations in postnatal mouse retinas have enabled recapitulation of retinal edema and neoangiogenesis without hyperglycemia. This article will review the experimental model systems utilizing mouse retinas and discuss perspectives on new drug discovery for the treatment of DR.
In DR, impairment of retinal vascular integrity causes elevated vascular permeability, which subsequently leads to fluid accumulation in extracellular spaces and swelling of retinal neurons and glial cells in the macula (Fig. 1A). Based on the postmortem examination of patients with DR, it was proposed that dropout of periendothelial mural cells (pericytes) from capillary walls might be an initial pathological event followed by deterioration of vessel integrity [8]. However, while DR clinically becomes manifest more than 5 years after the onset of diabetes, experimentally hyperglycemic rats or mice, with a 2-year average life expectancy, fail to represent human DR. Therefore, the widely-accepted 'pericyte dropout' concept had not been experimentally confirmed in diabetic animal models.
To overcome the limitation of diabetic animal models, we assessed the retinal vascular changes primarily caused by pericyte dropout in postnatal mouse retinas, in which new blood vessels start to grow just after birth [9,10]. In newly-formed retinal vessels, vascular endothelial cells (ECs) secrete platelet-derived growth factor (PDGF)-B to facilitate the proliferation and migration of pericytes that express PDGF receptor β (PDGFRβ) (Fig. 1B) [11-13]. While Pdgfb knockout mice demonstrate defective pericyte recruitment in brain capillaries [14], the perinatal lethality of mice genetically deficient for Pdgfb or Pdgfrb precluded observations of postnatal vascular development in retinas [15,16]. Thus anti-PDGFRβ monoclonal antibodies were administered daily intraperitoneally to neonatal mice. This pharmacological blockade of PDGFRβ signal did not affect the overall growth of the neonates, but successfully disrupted pericyte recruitment to developing retinal vessels (Fig. 1C) [17]. Remarkably, the retinal vasculature devoid of pericytes recapitulated most of the pathophysiological features characteristic of DR; i.e., disorganized vascular networks with vessel dilation and tortuosity, and progressive extravasations with retinal edema and hemorrhage. Electron microscopic observations of pericyte-free retinal vessels further depicted thickening of periendothelial basement membranes, which was also seen in human DR. These data, obtained by pharmacological PDGFRβ manipulations, together with EC-specific ablation of the Pdgfb gene [18], indicated that pericyte dropout was sufficient to reproduce retinal vascular abnormalities in DR, even without hyperglycemia.
In nascent vascular walls, pericytes contribute to stabilizing endothelial integrity via soluble signaling molecules and direct cell-cell contacts. In particular, angiopoietin-1 (Ang1) derived from pericytes binds to Tie2 receptor tyrosine kinase on EC surfaces, thereby activating downstream signals required for EC stabilization (Fig. 1B) [19,20]. Given that the absence of pericytes eliminates all of the pericyte-derived signals, we assessed to what extent Ang1 supplementation could restore the retinal vascular abnormalities caused by pericyte dropout. To our surprise, intraocular injections of recombinant Ang1 protein in conjunction with systemic injections of anti-PDGFRβ antibodies resulted in dramatic restoration of an organized architecture of retinal vascular networks [17]. Moreover, retinal edema and hemorrhage were completely suppressed despite the absence of pericytes (Fig. 1C). These experimental results indicated that Ang1 alone can maintain the structural integrity of retinal vessels in the complete absence of pericytes, and further suggested the potential efficacy of intraocular Ang1 therapy for the treatment of DME.
Although PDGF-B/PDGFRβ signal is a prerequisite for pericyte recruitment to growing retinal vessels, a PDGFRβ blockade failed to deplete pericytes in adult retinas. Thus, alternative signals other than PDGF-B/PDGFRβ might be involved in the maintenance or disruption of EC-pericyte association in DR. In the adult vasculature, Ang1 derived from pericytes constitutively phosphorylates Tie2 at a low level to maintain the mature phenotype of the endothelium [21]. In contrast to the stable Ang1 expression in quiescent vessels, Ang2, a natural antagonist of Ang1, is expressed predominantly in ECs of activated blood vessels (Fig. 1B) [22-24]. Because Ang2 binding does not activate Tie2 in ECs, it was proposed that Ang2 destabilizes EC-pericyte association by interfering with Ang1, thereby rendering ECs highly sensitive to the microenvironment [22]. Specifically, Ang2 promotes neoangiogenesis and vascular leakage in the presence of VEGF and proinflammatory cytokines, but facilitates vascular regression in the absence of VEGF [25]. Importantly, while Ang2 expression is upregulated by hypoxia and VEGF [26,27], high glucose directly upregulates Ang2 transcription in cultured ECs [28]. Thus, in diabetic patients, it is plausible that hyperglycemia might induce Ang2 expression in retinal ECs, thereby destabilizing the ECMC association. In this scenario, a pharmacological Ang2 blockade would be of therapeutic benefit for the prevention of pericyte dropout in DR. To date, several pharmaceutical companies have developed anti-Ang2 drugs, some of which are under clinical trial for the treatment of tumor angiogenesis [29]. In the near future, it is expected that the therapeutic potency of anti-Ang2 drugs will be clinically evaluated for the treatment of DR.
In accordance with the progression of DR, retinal capillaries are obstructed, generating nonperfused, ischemic retinal areas. In response to hypoxia, retinal neurons, and glial cells secrete a series of proangiogenic growth factors, including VEGF, which leads to the formation of new blood vessels from pre-existing ones. However, these new vessels do not grow into ischemic retinas, but grow out of the retinal surfaces, without resolving retinal hypoxia. Moreover, the extraretinal vessels directly cause vitreous hemorrhage and tractional retinal detachment, both of which severely impair vision. Therefore, for the prevention and regression of neoangiogenesis, laser photocoagulation is performed in an attempt to destroy hypoxic neurons thereby reducing oxygen demands [30]. More recently, intraocular anti-VEGF drugs have been administered prior to vitreous surgery to lessen the intraoperative and postoperative hemorrhagic complications [31]. Nevertheless, the ideal way to fundamentally resolve retinal ischemia would be to rectify the angiogenic directions into the hypoxic retinas, thereby regenerating functional capillaries. To develop such a vascular regeneration therapy, useful insight can be obtained from the procedures of intraretinal angiogenesis during development.
In the developing retinal vasculature, the angiogenic directions are determined by the endothelial filipodia projecting from the tips of sprouting blood vessels [11]. The tip ECs continuously project and retract numerous filopodia in response to the microenvironmental cues (Fig. 2A). The predominant force of endothelial filopodia projections is the concentration gradient of VEGF along the pre-existing networks of astrocytes, which migrate into the retina from the optic nerve prior to the onset of retinal vascular development [32]. In addition to VEGF secretion, astrocytes facilitate intraretinal angiogenesis by depositing extracellular matrices that serve as physiological scaffolds for migrating ECs [33]. As a consequence, developing vessels grow into hypoxic retinas in an organized fashion utilizing the astrocyte network as a template. Collectively, intraretinal angiogenesis largely depends on the signaling cues that regulate endothelial filopodia projections and the formation of matrix scaffolds by astrocytes (Fig. 2B).
During development, retinal astrocytes act as proangiogenic cells in response to hypoxia, which is corroborated by previous observations that retinal astrocytes are found only in species that develop vascularized retinas, such as humans and rodents [34,35], whereas deficits in astrocytes resulted in the failure of retinal vascular development in postnatal mice [36]. In unvascularized areas of developing retinas, astrocytes express VEGF and fibronectin, a major component of matrix scaffolds for migrating ECs that express α5β1 integrin. However, expression levels of these proangiogenic genes in retinal astrocytes are rapidly downregulated upon contact with growing blood vessels. This indicates that oxygenation or extrinsic signals derived from blood vessels might deprive retinal astrocytes of proangiogenic activities. Notably, in postnatal mouse retinas, an orphan nuclear receptor Tlx is expressed in proangiogenic astrocytes of unvascularized, but not vascularized, areas [37]. Furthermore, the expression levels of the Tlx and Vegf genes in astrocytes were synchronously controlled by the oxygen concentration in postnatal mouse retinas. In Tlx-deficient mice, gene expression, as well as the extracellular protein transport of fibronectin, was severely impaired in retinal astrocytes, which led to defects in intraretinal vascular growth [37]. These results together indicated that Tlx is a hypoxia-inducible proangiogenic switch that controls the formation of extracellular fibronectin scaffolds in retinal astrocytes (Fig. 2C).
In developing retinas, VEGF isoforms with positively-charged domains encoded by exons 6 and 7 bind to the heparan sulfate proteoglycans, thereby constructing concentration gradients along the astrocyte network [11,38]. On the other hand, soluble VEGF isoforms lacking the heparin-binding domain disturb VEGF concentration gradients. Indeed, overexpression of soluble VEGF isoforms leads to disoriented projections of endothelial filopodia [11,38]. To restrict the pathfindings of retinal vascular growth, a member of axon guidance molecules, including slit, netrin, and semaphorin, have been implicated in the retraction of disoriented endothelial filopodia [39]. Especially, a soluble Sema3E ligand, derived from the retinal neurons, mediates filopodia retraction by binding to the endothelial PlexinD1 receptor [40-42]. Because PlexinD1 expression is upregulated by VEGF in ECs of developing retinal vessels [40,41], it is postulated that Sema3E-PlexinD1 signals form a negative feedback loop against VEGF-induced filopodia projections in retinal ECs.
Projections and retractions of endothelial filopodia involve rearrangements of actin cytoskeletons and cell-matrix adhesions, which are regulated by Rho family small GTPases [43]. While Cdc42 promotes filopodia projections downstream of VEGF signals [44], RhoJ mediates endothelial filopodia retraction downstream of Sema3E signals [40]. Intriguingly, the activation states of Cdc42 and RhoJ were inversely regulated by VEGF and Sema3E (Fig. 2D). Because both Cdc42 and RhoJ bind to the Cdc42/Rac interactive binding domain of p21-activated kinases and neural Wiskott-Aldrich syndrome protein [45,46], it seems likely that Cdc42 and RhoJ compete for binding to their common downstream effector molecules, thereby controlling the dynamic rearrangements of actin cytoskeletons and cell-matrix adhesions. Given that Rho family small GTPases are activated by guanine nucleotide exchange factors (GEFs) [47], small molecule inhibitors targeting endothelial GEFs are expected to have therapeutic value for the control of endothelial filopodia projections. While a number of endothelial RhoGEFs have been identified [48], Arhgef15 acts as an EC-specific GEF to mediate VEGF-induced Cdc42 activation and further potentiates RhoJ inactivation, thereby promoting actin polymerization [49]. Because inactivation of the Arhgef15 gene resulted in retardation of retinal vascular growth [49], Arhgef15 would be a potential drug target for the treatment of aberrant neoangiogenesis in DR.
In an oxygen-induced retinopathy (OIR) model that represents neoangiogenesis in ischemic retinas [50], intraocular anti-VEGF drugs suppress not only the extraretinal neoangiogenesis but also the desirable regeneration of intraretinal vessels, resulting in the deterioration of retinal ischemia [40]. These experimental results raise caution for the clinical use of anti-VEGF drugs in the treatment of proliferative DR. In the OIR model, while Sema3E was expressed in retinal neurons, PlexinD1 and RhoJ were highly expressed in ECs of extraretinal vessels [40]. Because extraretinal neoangiogenesis was enhanced by inducible EC-specific Plxnd1 loss of function but was diminished by inducible EC-specific Rhoj gain of function [40], it seems likely that binding of neuron-derived Sema3E to endothelial PlexinD1 activates RhoJ, thereby suppressing extraretinal vascular outgrowth. By exploiting PlexinD1 in extraretinal vessels as a molecular target, it was further demonstrated that intraocular injections of Sema3E proteins suppressed aberrant neoangiogenesis without affecting intraretinal regeneration of functional capillaries (Fig. 2E) [40]. These results implied a clinical application of intraocular Sema3E therapy for the treatment of proliferative DR.
Experimental manipulations in developmental and ischemic retinal models in mice have provided invaluable information to understand the pathophysiology of retinal edema and neoangiogenesis. However, there remain a number of gaps to be filled between these animal models and human DR. For instance, the involvement of hyperglycemia in retinal vascular dysfunctions is yet to be elucidated. In the future, the combination of diabetic animal models with molecular manipulations will give further insights into the molecular and cellular events underlying the onset and progression of DR, by which new drug targets will successfully be determined.
Figures and Tables
Fig. 1
Impairment of retinal vascular integrity by pericyte dropout. (A) Transverse images of macular areas illustrated by optical coherence tomography. Note the fluid accumulation in diabetic macular edema (DME). Images courtesy of Akio Oishi, Kyoto University, Japan. (B) Interactions between endothelial cells (ECs) and pericytes (PCs) in newly formed blood vessels. EC-derived platelet-derived growth factor (PDGF)-B promotes proliferation and migration of PDGF receptor β (PDGFRβ)-expressing PCs, whereas PC-derived Ang1 stabilizes integrity of Tie2-expressing ECs. Ang2 acts as a natural antagonist for Ang1 in an autocrine fashion. (C) Restoration of retinal vascular integrity by Ang1 supplementation in the absence of PCs. H&E, hematoxylin and eosin staining; PECAM-1, platelet-endothelial cell adhesion molecule-1. Adapted from Uemura et al. J Clin Invest 2002;110:1619-28, with permission from American Society for Clinical Investigation [17].
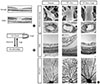
Fig. 2
Signaling cues and matrix scaffolds requisite for intraretinal angiogenesis. (A) High magnification of sprouting blood vessels in the postnatal mouse retina. Note the endothelial filopodia projections along the pre-existing astrocyte network. (B) Signaling cues regulating projections and retractions of endothelial filopodia. (C) Hypoxia-inducible response in retinal astrocytes during development. (D) Intracellular signaling cascades that regulate endothelial filopodia formation. (E) Selective suppression of extraretinal angiogenesis by intraocular Sema3E injections in the oxygen-induced retinopathy model. Note the exacerbation of retinal ischemia after the blockade of vascular endothelial growth factor (VEGF) signaling. EC, endothelial cell; VEGFR2, vascular endothelial growth factor receptor 2; PAK, p21-activated kinase; N-WASP, neural Wiskott-Aldrich syndrome protein. Adapted from Fukushima et al. J Clin Invest 2011;121:1974-85, with permission from American Society for Clinical Investigation [40].

ACKNOWLEDGMENTS
This work was supported by the Global COE program and Grants in Aid for Young Scientists (22689046) from MEXT, Japan.
References
1. Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012; 35:556–564.
2. Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012; 366:1227–1239.
3. Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen HV, Aiello LM, Ferrara N, King GL. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–1487.
4. Friedlander M. Fibrosis and diseases of the eye. J Clin Invest. 2007; 117:576–586.
5. Al-Latayfeh M, Silva PS, Sun JK, Aiello LP. Antiangiogenic therapy for ischemic retinopathies. Cold Spring Harb Perspect Med. 2012; 2:a006411.
6. Ho AC, Scott IU, Kim SJ, Brown GC, Brown MM, Ip MS, Recchia FM. Anti-vascular endothelial growth factor pharmacotherapy for diabetic macular edema: a report by the American Academy of Ophthalmology. Ophthalmology. 2012; 119:2179–2188.
7. Jain GK, Warsi MH, Nirmal J, Garg V, Pathan SA, Ahmad FJ, Khar RK. Therapeutic stratagems for vascular degenerative disorders of the posterior eye. Drug Discov Today. 2012; 17:748–759.
8. Cogan DG, Kuwabara T. The mural cell in perspective. Arch Ophthalmol. 1967; 78:133–139.
9. Uemura A, Kusuhara S, Katsuta H, Nishikawa S. Angiogenesis in the mouse retina: a model system for experimental manipulation. Exp Cell Res. 2006; 312:676–683.
10. Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007; 10:77–88.
11. Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003; 161:1163–1177.
12. Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998; 125:1591–1598.
13. Fruttiger M. Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci. 2002; 43:522–527.
14. Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997; 277:242–245.
15. Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994; 8:1888–1896.
16. Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994; 8:1875–1887.
17. Uemura A, Ogawa M, Hirashima M, Fujiwara T, Koyama S, Takagi H, Honda Y, Wiegand SJ, Yancopoulos GD, Nishikawa S. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. J Clin Invest. 2002; 110:1619–1628.
18. Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes HP, Shani M, Fassler R, Betsholtz C. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002; 21:4307–4316.
19. Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996; 87:1171–1180.
20. Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996; 87:1161–1169.
21. Wong AL, Haroon ZA, Werner S, Dewhirst MW, Greenberg CS, Peters KG. Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ Res. 1997; 81:567–574.
22. Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997; 277:55–60.
23. Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002; 3:411–423.
24. Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, Kutschera S, Bartels S, Appak S, Besemfelder E, Terhardt D, Chavakis E, Wieland T, Klein C, Thomas M, Uemura A, Goerdt S, Augustin HG. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest. 2012; 122:1991–2005.
25. Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009; 10:165–177.
26. Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem. 1999; 274:15732–15739.
27. Mandriota SJ, Pepper MS. Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res. 1998; 83:852–859.
28. Yao D, Taguchi T, Matsumura T, Pestell R, Edelstein D, Giardino I, Suske G, Rabbani N, Thornalley PJ, Sarthy VP, Hammes HP, Brownlee M. High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J Biol Chem. 2007; 282:31038–31045.
29. Cascone T, Heymach JV. Targeting the angiopoietin/Tie2 pathway: cutting tumor vessels with a double-edged sword? J Clin Oncol. 2012; 30:441–444.
30. Bressler NM, Beck RW, Ferris FL 3rd. Panretinal photocoagulation for proliferative diabetic retinopathy. N Engl J Med. 2011; 365:1520–1526.
31. Zhao LQ, Zhu H, Zhao PQ, Hu YQ. A systematic review and meta-analysis of clinical outcomes of vitrectomy with or without intravitreal bevacizumab pretreatment for severe diabetic retinopathy. Br J Ophthalmol. 2011; 95:1216–1222.
32. Watanabe T, Raff MC. Retinal astrocytes are immigrants from the optic nerve. Nature. 1988; 332:834–837.
33. Jiang B, Liou GI, Behzadian MA, Caldwell RB. Astrocytes modulate retinal vasculogenesis: effects on fibronectin expression. J Cell Sci. 1994; 107(Pt 9):2499–2508.
34. Schnitzer J. Retinal astrocytes: their restriction to vascularized parts of the mammalian retina. Neurosci Lett. 1987; 78:29–34.
35. Schnitzer J. Astrocytes in the guinea pig, horse, and monkey retina: their occurrence coincides with the presence of blood vessels. Glia. 1988; 1:74–89.
36. Fruttiger M, Calver AR, Kruger WH, Mudhar HS, Michalovich D, Takakura N, Nishikawa S, Richardson WD. PDGF mediates a neuron-astrocyte interaction in the developing retina. Neuron. 1996; 17:1117–1131.
37. Uemura A, Kusuhara S, Wiegand SJ, Yu RT, Nishikawa S. Tlx acts as a proangiogenic switch by regulating extracellular assembly of fibronectin matrices in retinal astrocytes. J Clin Invest. 2006; 116:369–377.
38. Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002; 16:2684–2698.
39. Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010; 2:a001875.
40. Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S, Uemura A. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest. 2011; 121:1974–1985.
41. Kim J, Oh WJ, Gaiano N, Yoshida Y, Gu C. Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev. 2011; 25:1399–1411.
42. Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005; 307:265–268.
43. Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008; 9:690–701.
44. Lamalice L, Houle F, Jourdan G, Huot J. Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene. 2004; 23:434–445.
45. Abe T, Kato M, Miki H, Takenawa T, Endo T. Small GTPase Tc10 and its homologue RhoT induce N-WASP-mediated long process formation and neurite outgrowth. J Cell Sci. 2003; 116(Pt 1):155–168.
46. Vignal E, De Toledo M, Comunale F, Ladopoulou A, Gauthier-Rouviere C, Blangy A, Fort P. Characterization of TCL, a new GTPase of the rho family related to TC10 and Ccdc42. J Biol Chem. 2000; 275:36457–36464.
47. Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007; 129:865–877.
48. Loirand G, Scalbert E, Bril A, Pacaud P. Rho exchange factors in the cardiovascular system. Curr Opin Pharmacol. 2008; 8:174–180.
49. Kusuhara S, Fukushima Y, Fukuhara S, Jakt LM, Okada M, Shimizu Y, Hata M, Nishida K, Negi A, Hirashima M, Mochizuki N, Nishikawa S, Uemura A. Arhgef15 promotes retinal angiogenesis by mediating VEGF-induced Cdc42 activation and potentiating RhoJ inactivation in endothelial cells. PLoS One. 2012; 7:e45858.
50. Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994; 35:101–111.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download