This article has been corrected. See "Erratum: Table Correction. AMPK and Exercise: Glucose Uptake and Insulin Sensitivity" in Volume 37 on page 155.
Abstract
AMPK is an evolutionary conserved sensor of cellular energy status that is activated during exercise. Pharmacological activation of AMPK promotes glucose uptake, fatty acid oxidation, mitochondrial biogenesis, and insulin sensitivity; processes that are reduced in obesity and contribute to the development of insulin resistance. AMPK deficient mouse models have been used to provide direct genetic evidence either supporting or refuting a role for AMPK in regulating these processes. Exercise promotes glucose uptake by an insulin dependent mechanism involving AMPK. Exercise is important for improving insulin sensitivity; however, it is not known if AMPK is required for these improvements. Understanding how these metabolic processes are regulated is important for the development of new strategies that target obesity-induced insulin resistance. This review will discuss the involvement of AMPK in regulating skeletal muscle metabolism (glucose uptake, glycogen synthesis, and insulin sensitivity).
AMPK is an evolutionary conserved αβγ heterotrimer consisting of an α catalytic subunit and βγ regulatory subunits. In mammals, all subunits are required for a stable complex and full activation of the enzyme [1]. AMPK is activated by low energy status (increased AMP/ADP: ATP) such as during exercise, and regulates metabolic process and energy homeostasis by switching off ATP consuming pathways (fatty acid and cholesterol synthesis) and switching on ATP generating processes (glucose uptake and fatty acid oxidation).
Skeletal muscle predominately expresses α2β2 complexes with low levels of α1 and β1. AMPK γ1 and γ2 are expressed in multiple tissues [2] whereas γ3 appears to be restricted to skeletal muscle [3-6], where it's association with α and β isoforms differ depending on the fiber composition of the muscle. In mice, γ3 and γ2 AMPK is predominately expressed in fast-twitch glycolytic extensor digitorum longus (EDL) muscle compared to slow-twitch oxidative soleus muscle [4,5] whereas in gastrocnemius muscle, γ1, γ2, and γ3 are evenly expressed [5]. In mice, both AMPK β1 and β2 associate with α2 in EDL whereas β1 AMPK is primarily associated with α2 AMPK complexes in soleus [7]. In humans, α1 and β1 are primarily expressed in slow-twitch oxidative [8] whereas γ3 is predominantly expressed in fast-twitch glycolytic muscle [9]. In human skeletal muscle, the majority of AMPK α2/β2 complexes associate with γ1, whilst the remaining α1/β2 and α2/β2 associate with γ3 [10]. See Table 1 for AMPK isoform heterotrimer distribution in EDL and soleus.
AMPK is inactive unless phosphorylated on the α-subunit activation loop at T172. Both AMP and ADP binding to the γ subunit enhances T172 phosphorylation and suppresses dephosphorylation [11-13]. Phosphorylation is the most potent activator of AMPK increasing activity >100 fold [14]. Upstream kinases, LKB1 and Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) phosphorylate AMPK T172 in mammalian cells. LKB1 is a heterotrimer complex with regulatory proteins STRAD and MO25 [15,16]. In skeletal muscle, studies in two independent models lacking LKB1 have shown that LKB1 is the major AMPK kinase in skeletal muscle [17,18]. Skeletal muscle-specific deletion of LKB1 (LKB1-MKO) from mice results in greatly reduced α2 AMPK T172 phosphorylation following activation by AICAR (a cell-permeable adenosine analog that can be phosphorylated to form 5-aminoimidazoel4-carboxamide-1-d-riborfuronosil-5'monophosphate) or muscle contractions/exercise [17-19]; however, AMPK α1 activity does not appear to be substantially reduced in these models suggesting an alternative upstream kinase may regulate AMPK α1 activity. CaMKK activates AMPK in response to elevated intracellular Ca2+ [20-22]; however, there is a lack of genetic evidence supporting the importance of this kinase in regulating skeletal muscle AMPK T172 phosphorylation.
Skeletal muscle constitutes around 45% of lean body mass and is a highly metabolic tissue that is a major contributor to whole-body expenditure. Skeletal muscle is responsible for around 80% of insulin-stimulated glucose disposal [23-25]; therefore, responsiveness to insulin in this tissue is important for regulating whole-body insulin sensitivity. Over the past decade, reduced physical activity levels, due to increasing technologies that promote a sedentary lifestyle and the overconsumption of food, has led to an epidemic of obesity and type 2 diabetes. Obesity and type 2 diabetes are casually linked through their association with skeletal muscle insulin resistance. Insulin resistance is characterized by the reduced responsiveness of target tissues (e.g., skeletal muscle, liver and adipose tissue) to insulin. This results in compensatory hyperinsuliemia, but hyperglycaemia and hyperlipidemia due to the reduced ability of insulin to suppress liver gluconeogenesis and promote glucose uptake in skeletal muscle. Insulin also promotes glucose uptake into adipose tissues and suppresses lipolysis. Therefore, understanding the underlying mechanisms that contribute to the development of insulin resistance as well the pathways involved in improving insulin sensitivity are critical for the development of new strategies (both pharmacological and non-pharmacological such as exercise) that target obesity-induced insulin resistance. Activation of AMPK by pharmacological means have supported a role for AMPK in regulating glucose uptake, fatty acid oxidation, mitochondrial oxidative capacity and insulin sensitivity; therefore, AMPK may be potential target for the treatment of obesity induced insulin resistance given these processes are reduced in skeletal muscle from obese and type 2 diabetic humans and rodents.
Whole-body α1, α2 [26], β1 [27], β2 [28,29], and γ3 [4] KO and muscle-specific β1β2 KO (β1β2 M-KO) [30] mice as well as transgenic mice overexpressing a kinase dead form of α2 AMPK in skeletal muscle (KD or α2iTg) [31,32] have been generated to help define the role of AMPK in regulating skeletal muscle metabolism. These studies have investigated the role of AMPK in regulating carbohydrate (glucose uptake and glycogen synthesis) and lipid (fatty acid uptake and oxidation) metabolism, and mitochondrial biogenesis and insulin sensitivity at rest and during or following exercise and muscle contractions.
AMPK α2 KO and α2KD mice have reduced AMPK T172 phosphorylation and α2 activity in skeletal muscle, but enhanced or modestly reduced α1 activity, respectively [31,33,34]. In contrast, AMPK α1 KO mice have normal AMPK T172 phosphorylation in skeletal muscle despite reduced α1 activity, showing that α2 AMPK is the predominant isoform contributing to AMPK activity in skeletal muscle [35]. The role of AMPK in insulin sensitivity has been extensively studied in AMPK deficient models fed either a chow or high-fat diet, the latter being considered the most physiological model to replicate human obesity and insulin resistance in rodent studies. AMPK α1 KO and α2KD mice have normal insulin sensitivity on a chow and high-fat diet [33,36]. However, an alternative muscle-specific mouse model on a model FVB background, rather than a C57Bl6 background, overexpressing a muscle-specific KD AMPKα2 B157A mutation (α2iTg), are glucose intolerant and develop exacerbated skeletal muscle insulin resistance when fed a high-fat diet for 30 weeks, which occurs independently of an increase in muscle lipids [37]. In this study, high-fat fed α2iTg mice had reduced IRS1 and Akt protein expression in skeletal muscle compared to WT counterparts, which may have contributed to the reduced skeletal muscle glucose uptake in response to insulin [37]. The FVB strain is known to be resistant to obesity-induced insulin resistance and given that the greater glucose intolerance in α2iTg mice was not evident until after 26 weeks on the high-fat diet, it is unlikely that AMPK is directly involved. Whole-body AMPK α2 KO mice have reduced insulin secretion and whole-body insulin-stimulated glucose utilization due to elevated catecholamine levels and a hyperactive sympathetic nervous system [33]. Basal and insulin-stimulated glucose uptake in isolated muscles is normal in AMPK α2 KO mice, further supporting that insulin resistance was caused by an external defect independent of skeletal muscle AMPK.
Whole-body deletion of both β1 and β2 is embryonic lethal [38]. AMPK β1 KO mice have reduced AMPK activity in liver but not skeletal muscle, are hyperphagic and as a result have lower body weight, adiposity and hepatic lipid accumulation, and are protected from high-fat diet induced insulin resistance [27]. In contrast, AMPK β2 KO mice have reduced AMPK activity in skeletal muscle but not liver or adipose, and have a compensatory increase in AMPK β1 protein expression and maintained α1 expression in skeletal muscle [28]. AMPK β2 KO mice are characterized by reduced exercise capacity and increased susceptibility to develop insulin resistance on a high-fat diet possibly due to greater ceramide accumulation in skeletal muscle [28]. Interestingly, lipid accumulation occurred in AMPK β2 KO mice despite intact fatty acid oxidation rates and increased glycogen utilization in skeletal muscle [28]. An alternative AMPK β2 KO mouse model has recently been generated and these mice have a similar phenotype the β2 KO model reported by Steinberg et al. [28], but in addition, show that AMPK plays an important role in the adaptive response to fasting whereby AMPK β2 KO mice have reduced phosphorylation of S6K and the AMPK substrate ULK1, which is involved in autophagy [29,39].
We have recently generated muscle-specific AMPK β1β2 double KO mice (β1β2M-KO) to alleviate concerns related to residual AMPK activity due to remaining subunit homologs being present or upregulated in previous AMPK deficient models (α2 KO, α2KD, β2 KO, γ3 KO). As anticipated, AMPK β1β2M-KO mice have undetectable levels of AMPK activity at rest or during exercise [30]. Their phenotype is more dramatic compared to whole-body AMPK β2 KO mice [28] and is characterized by dramatically reduced voluntary wheel activity (75%), maximal exercise capacity (95%), mitochondrial content (30%) and exercise-mediated glucose uptake in skeletal muscle (55% to 70%) [30]. Interestingly, young (9 to 12 weeks) AMPK β1β2M-KO mice have similar whole-body weight and adiposity, and consistent with this, whole-body and skeletal muscle specific insulin sensitivity is similar when compared to wild-type littermates [30]. These studies highlight an important role for AMPK in regulating exercise capacity, mitochondrial content and glucose uptake during exercise, but not insulin sensitivity.
The γ1 subunit is ubiquitously expressed similar to γ2; however, γ2 expression is predominant in heart [2,3]. In contrast, γ3 appears to be expressed specifically in skeletal muscle [3-6]. Studies from both whole-body γ3 KO and transgenic γ3 overexpressing or gain-of-function mice have revealed an important role for the γ3 subunit in regulating glucose uptake, glycogen synthesis, fatty acid oxidation and mitochondrial biogenesis in skeletal muscle [40]. Whole-body γ3 KO mice have reduced AMPK activity at rest, during exercise and in response to fasting, but normal insulin sensitivity under basal conditions and glucose uptake during exercise [4,41]. Interestingly, gain-of-function mutations in the γ subunits result in glycogen storage disease in humans [42-44] and pigs [45]. AMPK γ3 R225Q mutation is the most studied mutation and this mutation is homologous to R225Q in Hampshire pigs, as well as AMPK γ2 R200Q in humans, which is associated with Wolff-Parkinson-White syndrome [46,47]. Interestingly, transgenic mice harboring the R225Q mutation in AMPK γ3 subunit have higher AMPK activity and glycogen levels and are protected from diet-induced insulin resistance [4], which is a likely effect of increased fatty acid oxidation and subsequent reduction in skeletal muscle lipids. Mice with an AMPK γ3 R225Q mutation are also fatigue resistant during exercise whereas AMPK γ3 null mice are prone to fatigue during exercise and have impaired glycogen re-synthesis following exercise (see section AMPK and glycogen synthesis) [4,48]. Whilst γ3 appears to be predominantly expressed in glycolytic muscles, training reduces γ3 expression in humans [10,49], which may be an adaptive response to enhanced oxidative capacity (preference for lipid oxidation). These studies suggest that targeting AMPK may enhance exercise performance and be protective against insulin resistance disease development in obesity.
An important feature of skeletal muscle is its ability increase ATP turnover (>100 fold) during exercise in order to provide energy for contracting muscles [50]. Under such conditions, AMP and ADP levels are rapidly increased in an intensity-dependent manner and ATP levels decline slightly. AMPK is a sensor of cellular energy status that is activated by AMP and ADP. Consistent with this, AMPK is activated during exercise in both rodents and humans [51-60], in an intensity-dependent manner [57,59-61]. Both AMPK α1 and α2 are activated during exercise; however, α1 activity appears to be increased during high intensity muscle contractions, which are equivalent to >100% maximal oxygen consumption (VO2 Max; measure of maximal exercise capacity) [59,62,63]. In contrast, α2 activity is increased at low intensity exercise starting at 40% VO2 Max and increases progressively with exercise intensity.
Exercise can also regulate glucose uptake by an insulin-independent mechanism involving AMPK [30]. Importantly, GLUT4 translocation during exercise is normal in skeletal muscle of type 2 diabetics [64], and exercise also improves insulin sensitivity. For these reasons, exercise is often advised for both the prevention and treatment of insulin resistance. Therefore, understanding the underlying mechanisms regulating glucose uptake is an important area of research.
The first step in understanding how glucose uptake is regulated during exercise and how insulin sensitivity is enhanced following exercise is to understand how insulin regulates glucose uptake in normal healthy individuals. In brief, insulin promotes glucose uptake through a signaling cascade consisting of a number of spatially distinct phosphorylation events that result in moving glucose transporters (GLUT4) to the plasma membrane, which upregulates glucose transport into the cell (Fig. 1). Specifically, insulin mediates glucose uptake by binding to its tyrosine kinase receptor on the outside of the cell, causing further activation/phosphorylation of the IRS proteins-1 and 2 inside the cell. Activation of IRS proteins trigger the activation of class I phosphatidlyinositol-3-kinase (PI3K) and subsequent interaction with p110 catalytic and p85 regulatory subunits via increased interaction with SH2 domains, which allows phosphorylation of phosphatidylinositol 4,5-bisphosphate (PIP2) to form phosphatidylinositol 3,4,5-triphosphate (PIP3) at the plasma membrane. This promotes Akt recruitment to the plasma membrane via PH domain interaction with PIP3, which induces a conformational change in Akt that allows phosphorylation at T308 S473 and activation [65]. Interaction with the activating kinase phosphoinositide-dependent kinase (PDK) phosphorylates T308 in the activation loop and leads to partial activation of Akt/protein kinase B (PKB) [66]. The complete activation of Akt requires phosphorylation of Akt at the hydrophobic C-terminal domain S473 residue by mammalian target of rapamycin complex 2 (mTORC2) [67]. Akt phosphorylation of TBC1 (tre-2/USP6, BUB2, cdc16) domain family, member 4 (TBC1D4) (formerly known as Akt substrate of 160 kDa; AS160) at T642 and phospho S/T Akt (PAS) sites, with the (R/K)X(R/K)XXS*/T* recognition motif, is important for GLUT4 translocation in response to insulin. Phosphorylation of TBC1D4 increases binding to 14-3-3 proteins [68-70], and this has been proposed to inhibit RabGAP function towards downstream Rabs; thereby, promoting GTP loading and activation of Rabs and the release of glucose transporter GLUT4 from intracellular compartments to the plasma membrane for glucose uptake [69,71-73].
Correlation studies suggest that T642 (human and T649 in mouse) is the predominant Akt site on TBC1D4 recognized by the PAS antibody [74]. In mice, whole-body mutation of S642 (S649 in mouse) to alanine (TBC1D4 T649A KI) reduces whole-body glucose and insulin intolerance and impairs insulin-stimulated glucose uptake and GLUT4 translocation in muscle [75]. Similarly, overexpression of TBC1D4 by mutation of four predominant Akt phosphorylation sites (S318, S588, T642, and S751) (4P mutant) in mouse skeletal muscle inhibits insulin-stimulated glucose uptake in vivo by ~50% [76]. Interestingly, this defect is rescued by introduction of a mutant inactive Rab/GAP (R/K), suggesting that this domain is essential for insulin-stimulated glucose uptake. In human skeletal muscle, Treebak et al. [77] have shown that using site-specific antibodies against S318, S341, S588, and S751, TBC1D4 phosphorylation is increased following insulin stimulation during a hyperinsulinemic clamp. These studies support that phosphorylation of TBC1D4 and subsequent 14-3-3 binding and deactivation of this RabGAP is important for insulin-stimulated glucose uptake. It has been proposed that AMPK may phosphorylate TBC1D4 at T642 and promote 14-3-3 binding; however, this has not been confirmed in studies using various cell lines (L6 myotubes, 3T3L1 adipocytes) and cell free assays [70,78-80]. TBC1D4 may also play a role in the insulin sensitizing effects of exercise [77], as exercise training has been shown to restore TBC1D4 phosphorylation in skeletal muscle from type 2 diabetics [81].
Akt2 is essential for TBC1D4 T642 and PAS phosphorylation in response to insulin [76]. Akt2 KO mice and siRNA targeted silencing of IRS1 and Akt2 in human myotubes have blunted insulin-stimulated TBC1D4 phosphorylation compared to their homologous counterparts IRS2 and Akt1 and 3 [68,82]. Reduced IRS1 and Akt phosphorylation has previously been considered an important component leading to reduced insulin-stimulated glucose uptake that is associated with insulin resistance. However, recent evidence suggests that low levels of Akt activation are sufficient for maximal GLUT4 translocation in response to insulin [83], and modest reductions in IRS1 do not necessarily translate into impaired insulin action in skeletal muscle, which has been highlighted in IRS1 heterozygous mice [84] and shRNA mediated silencing of IRS1 in vivo that display normal glucose uptake in response to insulin [85]. A further explanation for these observations whereby insulin signaling is normal despite reduced insulin-stimulated glucose uptake is that insulin-stimulated glucose uptake can also occur independently of PKB/Akt signaling [86]. This process still requires PI3K but involves activation of Rac1, a small GTPase protein from the Rho family that promotes glucose uptake by association with actin; thereby promoting actin remodeling [87].
GLUT4 vesicles move along cytoskeletal elements that are directed by GTP-bound (active) Rab-proteins; small G proteins required for membrane trafficking [69]. Actin remodeling is a dynamic multistep process that occurs at the cell surface, and changes in the spatial and temporal arrangement of actin are considered important for providing a scaffold for the transmission of signaling from insulin receptor to GLUT4. Whilst the regulation of actin by Rac1 and the interaction between actin and GLUT4 vesicles in regulating insulin-stimulated glucose uptake has not been extensively studied compared to the Akt/PKB pathway, it is known that that Rac1 is rapidly activated (GTP loaded) in response to insulin in both adipose and muscle cells, and a dominant-negative Rac mutant unable to bind GTP prevents insulin-induced actin remodeling [88]. Evidence that the Rac/actin and PKB are independent pathways both regulating glucose uptake in response to insulin is supported by studies showing that 1) siRNA knockdown of Rac1 in L6 myotubes reduces insulin-stimulated GLUT4 translocation without altering Akt phosphorylation [89]; 2) inhibition of P13K with wortmannin prevents actin remodeling [90] and in contrast, 3) overexpression of a dominant negative Akt mutant does not alter actin remodeling in L6 myotubes [91]. Few studies have investigated the involvement of the Rac/actin pathway in insulin resistance; however, ceramides, which are bioactive lipids that are elevated in obese skeletal muscle and reduce insulin-stimulated glucose uptake, reduce insulin-stimulated actin remodeling and GLUT4 translocation in L6 myotubes [89]. PKC has also been implicated in regulating GLUT4 trafficking to the plasma membrane in response to insulin; however, PKC appears to lie downstream of Rac [92].
Recently, TBC1D1, a closely related paralog of TBC1D4, has also been identified as an Akt substrate that may play a critical role in regulating GLUT4 trafficking events. TBC1D1 is a candidate gene for the development of severe familial obesity in females, due to the presence of an R125W coding variant that has been identified in 4p15-14-linked obesity pedigrees from American and French populations [93,94]. It is not currently known how the R125W mutation increases susceptibility to obesity; however, it's localization in the phosphotyrosine binding domain of the N-terminus may affect TBC1D1 function [93]. Expression of the R125W mutation in mice reduces insulin-stimulated glucose uptake in skeletal muscle [95]. The C-terminus of TBC1D1 includes a TBC (Tre-2/BUB2/Cdc16) domain that contains Akt, AMPK, and PKA phosphorylation sites thought to be important for regulating Rab GAP activity and association with Rab GPTases; however, the R125W mutation does not affect TBC1D1 PAS phosphorylation [93,95], which suggests that they have distinct functions in regulating glucose uptake. Silencing TBC1D1 in L6 muscle cells by siRNA increases basal and insulin-stimulated GLUT4 translocation [71], and overexpression of TBC1D1 in 3T3-L1 adipocytes with low endogenous TBC1D1 expression inhibits insulin-stimulated GLUT4 translocation, which is reversed by expression of a mutated form of TBC1D1 whereby the GAP domain has been inactivated [96]. Recombinant congenic mice lacking TBC1D1 are protected against diet-induced obesity, which is likely due to increased fatty acid uptake and oxidation in skeletal muscle [97]. These studies support a role for TBC1D1 in regulating glucose uptake.
TBC1D1 and TBC1D4 share 47% sequence identity; however, their GAP domains are 79% identical and 91% similar [96]. Like TBC1D4, TBC1D1 exerts a regulatory role in glucose uptake and metabolism through regulation of its GAP activity. In contrast to TBC1D4, TBC1D1 is only phosphorylated on one of the five standard Akt motifs (T590) and one partial one (S501), which corresponds to T649 and S577 of TBC1D4 in mouse, respectively. The PAS antibody recognizes T590 on TBC1D1, which is the equivalent T642 site on TBC1D4. It has been predicted that this site may also be an AMPK site [74]. Unlike TBC1D4, 14-3-3 binding to TBC1D1 does not occur upon insulin stimulation [70]. TBC1D4 and TBC1D1 also differ in their expression in tissues, whereby TBC1D1 is predominately expressed in skeletal muscle with little in adipose and none in heart whereas TBC1D4 is ubiquitously expressed [98]. Of note, TBC1D4 is predominately expressed in oxidative soleus muscle, whereas TBC1D1 is more highly expressed in glycolytic muscle such as tibialis anterior and extensor digitorum longus [98]. The predominant expression of TBC1D4 in oxidative soleus muscle compared to TBC1D1 may be related to its predominant role in regulating insulin-stimulated glucose uptake, which would be consistent with greater insulin binding capacity of oxidative muscles.
A number of additional Akt phosphorylation sites on TBC1D1 have also been identified (see [99]), and mutation some of these sites (T499, S489, S501, and T590) to alanine in C2C12 myotubes reduces GLUT4 translocation to the cell surface in response to insulin; however, S501 and T590 appear to be the most predominant regulators of TBC1D1 GAP activity controlling this process [99]. Interestingly, the combination of AICAR with insulin is additive in relieving TBC1D1 inhibition on GLUT4 translocation in both wild-type and mutant cells compared to each treatment alone, suggesting a possible role for AMPK in the regulation of TBC1D1 activity [99]. In contrast to the above findings, An et al. [95] has shown that overexpression of TBC1D1 by mutation of four potential AMPK/Akt phosphorylation sites (4P) (S231, T499, T590, S621) in skeletal muscle does not impair insulin-stimulated glucose uptake in vivo, suggesting that alternative AMPK sites may be important or AMPK does not play a role in regulating insulin-stimulated glucose uptake via TBC1D1. In support of this, insulin also does not increase AMPK T172 or ACC S79/212 phosphorylation [74] and AMPK α2, β2 and γ3 null and α2KD and β1β2 M-KO mice have normal insulin-stimulated glucose uptake in muscle [26,28,30,36,100] despite blunted TBC1D1 PAS and T590 phosphorylation in response to insulin [30,74].
Under basal conditions, a role for AMPK in regulating TBC1D1 has been suggested given that 1) the Akt inhibitor wortmannin does not alter basal TBC1D1 phosphorylation [74,101]; 2) Akt2 KO mice have normal basal TBC1D1 phosphorylation [102]; and 3) AMPK α2 KD, α2 KO α2iTg and β1β2 M-KO mice have reduced basal TBC1D1 phosphorylation [30,74,102]. Collectively, these studies suggest that whilst Akt is important for mediating insulin-induced TBC1D1 phosphorylation, AMPK activity is important for maintaining basal levels of T590 phosphorylation. In further support of this, it has been shown that TBC1D1 co-localizes with GLUT4 in response to A-769662 in L6 myotubes [70]. Based on these findings, AMPK may be important for TBC1D1 localization and priming in order to allow phosphorylation by Akt following insulin stimulation. Further investigation on TBC1D1 localization in muscle-specific AMPK β1β2-KO myotubes in response to different stimuli would be of interest for determining this.
Like insulin, exercise increases the rate of glucose uptake into contracting skeletal muscles, by a process regulated by GLUT4 translocation to the plasma membrane and transverse tubules. Interestingly, insulin and exercise/contractions regulate glucose uptake in skeletal muscle by distinct mechanisms (Fig. 2). This revelation came from the following original observations:
1) PI3 kinase inhibitor wortmannin inhibits insulin but not contraction-stimulated glucose uptake. Contraction does not promote IRS-1 autophosphorylation or increase PI3 kinase activity [103-106].
The involvement of TBC1D4 in regulating glucose uptake in response to contraction has been extensively summarized (see review [73]). In brief, evidence that TBC1D4 is involved in contraction-mediated glucose uptake came from the following studies:
1) Rat epitrochlearis muscle contracted in vitro in the absence of insulin increased TBC1D4 phosphorylation [115].
2) Contraction and insulin additively increased TBC1D4 phosphorylation [115].
3) TBC1D4 4P mutant has impaired contraction-mediated glucose uptake [68].
AMPK rather than Akt has been proposed to be a possible candidate regulating TBC1D4 activity in response to exercise and muscle contractions given 1) Akt phosphorylation is not increased by exercise; 2) AICAR stimulates glucose uptake independently of insulin; 3) AICAR increases phosphorylation of TBC1D4 at PAS motifs in both rat [115] and mouse skeletal muscle [76]; and 4) glucose uptake is impaired in the TBC1D4 4P mutant in response to AICAR and contraction [76]. The binding of Ca2+/calmodulin to the Ca2+/calmodulin binding domain (CBD) of TBC1D4 also regulates glucose uptake in response to exercise but not insulin. However, combining the 4P mutant with a CBD mutant results in similar reductions in glucose uptake when compared to the 4P and CBD mutants alone [116], suggesting that each is necessary but not sufficient for regulating this process.
Alternative studies provide evidence that PAS-TBC1D4 phosphorylation is not essential for contraction-mediated glucose uptake given that increases in phosphorylation do not appear at the onset of exercise when glucose uptake is rapidly increased (reviewed in [73]). Consistent with this, TBC1D4 T649A KI mice do not have impaired AICAR and contraction mediated glucose uptake, suggesting that the other three sites (S325, S595, and S758) may also be important for regulating TBC1D4 activity and glucose uptake during exercise [78]. Of note, TBC1D4 PAS phosphorylation is not increased with contraction in TBC1D4 T649A KI mice, supporting that TBC1D4 PAS antibody primarily recognizes the T649 site. Worth mentioning is that the S595 site has also been shown to be a potential AMPK site [80], and indeed, its phosphorylation was increased upon in situ contraction in muscle extracts from both wild-type and TBC1D4 T649A KI mice [78]. Further studies in alternative knockin mutant mice may be important for further defining the role of TBC1D4 in regulating glucose uptake in response to exercise and contraction.
Like TBC1D4, TBC1D1 is phosphorylated in response to AICAR and muscle contractions/exercise [74,95,101,102,117]. In response to muscle contractions, increases in TBC1D1 phosphorylation are important for 14-3-3 binding, inhibition of GAP domain function and subsequent increases in glucose uptake [95,102]. This is in contrast to TBC1D4 whereby 14-3-3 binding is not considered important for regulating glucose uptake during exercise [78]. AMPK phosphorylates multiple sites on TBC1D1 (S231, S499, T590, S660, S700), and muscle contractions/exercise predominately induces TBC1D1 phosphorylation on S231 in both humans and mice [74,102,117]. TBC1D1 T590 is also predominately phosphorylated in response to muscle contractions [74]; however, this has not been observed in all studies [102]. Interestingly, two separate studies inactivating 4 predicated TBC1D1 phosphorylation sites (4P mutant) results in 25% to 30% reduction in contraction-mediated glucose uptake [95,102]. An et al. [95] mutated conserved Akt site (T596) and 3 predicted AMPK sites (S231, T499, S621) whereas Vichaiwong et al. [102] mutated 4 predicted AMPK sites (S231, S499, S660, S700).
TBC1D1 expression is normal in skeletal muscle from type 2 diabetics [99], suggesting a possible explanation for normal glucose uptake during exercise in these subjects. In contrast, TBC1D1 expression is reduced by ~50% in skeletal muscle of AMPK α2 KO, α2 KD, α2iTg and β1β2 M-KO mice [30,74,102,117], and this may be compensatory for the lack of phosphorylation and inhibition by AMPK, or alternatively, reduced muscle glucose uptake in AMPK β1β2 M-KO mice. TBC1D1 PAS phosphorylation is slightly increased in α2iTg and α2KO mice but unresponsive in AMPK KD and β1β2 M-KO mice following contractions [30,74], suggesting that AMPK is important for PAS phosphorylation in response to contraction. However, the effect of AMPK on regulating glucose uptake during exercise is less clear. AMPK α2 KO and α2 KD and α2iTg mice have normal or only modest reductions in glucose uptake during contractions [4,26,31,32,118-120]. It appears that only when AMPK activity is completely abolished, as in β1β2 M-KO mice, that more dramatic reductions in glucose uptake are observed [30], which are similar to that observed in LKB1-MKO mice [18]. Although TBC1D1 expression or PAS phosphorylation was not assessed in skeletal muscle from β2 or γ3 KO mice, contraction-mediated glucose uptake was also normal [4,28], suggesting that the remaining subunit isoforms (likely contributing to maintained or elevated α1 AMPK activity) was sufficient to allow for normal glucose uptake. In addition to AMPK, the AMPK-related kinase SNARK has also been shown to be important for glucose uptake during contraction of mouse skeletal muscle [121]. Collectively, these studies support a role for AMPK and possibly AMPK-related kinases in regulating glucose uptake during exercise and muscle contractions. Alternative unidentified pathways may also exist and further studies are required to fully define the underlying mechanisms regulating GLUT4 translocation in response to exercise.
Exercise enhances insulin-stimulated glucose uptake, an effect that can persist for hours following an acute bout of exercise. The underlying mechanisms responsible for exercise-induced improvements in insulin sensitivity are not currently known; however, a number of possible candidates have been suggested (including TBC1D4, AMPK, interleukin 6 [IL-6], adiponectin, glycogen synthesis/levels, fiber-type/oxidative capacity). Arias et al. [122], was one of the first to show that TBC1D4 PAS phosphorylation was elevated in rat skeletal muscle following an acute bout of exercise, an effect that persisted for 3 to 4 hours postexercise. These studies lead to the idea that TBC1D4 may be involved in the insulin sensitizing effects of exercise. Since then, studies comparing insulin sensitivity following both in vivo exercise and in vitro contraction of isolated muscles have been performed. These studies have found that whilst TBC1D4 T642 and PAS phosphorylation is increased immediately after exercise and 3 hours postexercise consistent with enhanced insulin-stimulated glucose uptake; following in vitro contractions TBC1D4 phosphorylation had returned to resting levels 3 hours postexercise despite having an additive effect on glucose uptake in response to insulin [123]. It was proposed that circulating factors such as IL-6 may be involved in postinsulin sensitizing effects of exercise; however, TBC1D4 phosphorylation still returned to resting levels even when serum was added to the incubation media or not. These studies indicate that whilst circulating factors may also play a role in the insulin sensitizing effects of in vivo exercise (see IL-6 and adiponectin section); unknown TBC1D4-independent mechanisms are also involved following in vitro contractions. Studies in humans have also revealed similar findings, whereby 60 minutes of leg extension exercise (80% of maximal exercise capacity) increases TBC1D4 phosphorylation on specific sites (S318, S341, S588, and S751) but not PAS or T642 phosphorylation 4 hours post exercise [77]. In this study, Treebak et al. [77] also found that 14-3-3 binding to TBC1D4 in skeletal muscle was not further enhanced by leg exercise when compared to insulin alone despite increased insulin-stimulated glucose uptake following exercise, suggesting that insulin sensitivity following exercise may not involve 14-3-3 binding to TBC1D4. Similarly, Howlett et al. [124] found that although 3 hours of cycling increased 14-3-3 binding to TBC1D4 immediately following exercise, this had returned to baseline despite maintained TBC1D4 phosphorylation 3 hours postexercise. Whilst these studies support a role for TBC1D4 in insulin sensitivity following exercise, it also suggests that alternative mechanisms are also involved.
Unlike TBC1D4, TBC1D1 requires 14-3-3 binding in response to contraction and AICAR but not insulin in both human and rodent skeletal muscle [74,117]. TBC1D1 phosphorylation is enhanced immediately following exercise but returns to basal levels 3-4 and 27 hours post-exercise in rats, suggesting that the involvement of TBC1D1 in the insulin sensitizing effects of exercise are different from that of TBC1D4. Both acute exercise and AICAR activate AMPK and increase insulin sensitivity. These findings have led to the idea that AMPK may be involved in the insulin sensitizing effects of exercise. Phosphorylation of TBC1D1 at S237 appears to be the predominant site phosphorylated by AMPK; however, TBC1D1 PAS phosphorylation does not appear to correlate well with S237 phosphorylation [74]. No studies have assessed the TBC1D1 phosphorylation at specific sites following exercise or investigated insulin sensitivity in TBC1D1 4P mutant, but these studies would provide valuable evidence about the involvement of AMPK and TBC1D1 in the insulin sensitizing effects of exercise.
Glycogen synthesis is increased following exercise and during insulin stimulation, and insulin-stimulated glycogen synthesis is enhanced following exercise. Contraction and insulin promote glycogen synthesis by distinct mechanisms but both involve multisite dephosphorylation and activation of glycogen synthase (GS). In the case of insulin-stimulated glycogen synthesis, insulin signaling thru Akt inactivates GSK3 by phosphorylation at S9 and 21, and this in turn activates GS. In contrast, contraction requires protein phosphatase 1 (PP1) regulatory unit R(GL) to activate GS [125]. GS is phosphorylated on at least nine sites and insulin and contraction dephosphorylates both C- and N terminal sites [126]. Insulin-stimulated glucose transport enhances cellular G6P, which in turn promotes glycogen synthesis. Mutation of the G6P allosteric binding site on GS R582 to alanine (GS R582 KI) in mice reduces glycogen synthesis in response to insulin, suggesting that the G6P allosteric control of GS is also important in controlling glycogen synthesis [127]. Similarly, mutation of two Akt phosphorylation sites on GSK3 (S9 and S21) to alanine prevents inhibition of GSK3 and results in impaired insulin-stimulated glycogen synthesis [128]. In regards to AMPK, the AMPK β subunits contain a central domain, referred to as the carbohydrate-binding motif (CBM) or glycogen-binding domain that allows AMPK to bind to glycogen [129]. This domain is highly conserved between isoforms and across species, ranging from mammals, fruitfly (Drosophila melanogaster), worm (Caenorhabditis elegans), yeast (Saccharomyces cerevisiae), and plants [129]. The CBM is closely related to isoamylase domains found in glycogen and starch branching enzymes [129]. Recently, mutations in the CBM have been shown to prevent glycogen binding and reduce total AMPK activity in cell-free assays [130] and in vivo [131]. Glycogen can also inhibit AMPK, suggesting that AMPK may regulate cellular energy status through glycogen sensing [130].
AMPK regulates glycogen synthesis through phosphorylation of glycogen synthase (GS) at site 2 (S7) [132]. This inhibits enzyme activity in isolated muscles treated with AICAR [133-135]. In liver, GS inactivation by AMPK activators (AICAR and A769662) occurs at S7 phosphorylation site on GS2, which is the liver-specific isoform [136]. Interestingly, AMPK activation by AICAR promotes glucose uptake and increases G6P, which can be used for glycogen synthesis or metabolized through glycolysis to produce ATP [127]. Recently, acute treatment with AICAR was shown to promote glycogen synthesis in skeletal muscle through a G6P-GS dependent pathway; an effect that is blunted in GS R582A KI mice [137]. These studies suggest that GS inhibition by AMPK can be overridden by G6P. LKB1-MKO and AMPK α2 KO, β2 KO and α2 KD mice have lower starting glycogen levels in skeletal muscle [17,138-140], supporting a role for AMPK in glycogen synthesis.
It was previously thought that reduced glycogen synthesis in skeletal muscle from type 2 diabetics contributes to reduced insulin-stimulated glucose uptake [141]. Exercise promotes glycogen resynthesis and this may be important for improving insulin sensitivity postexercise. Funai et al. [142] found that refeeding rather than fasting following exercise reduced the enhanced effects of insulin on glucose uptake as well as TBC1D4 T642 and PAS phosphorylation. These studies suggests that whilst increases in insulin-stimulated glucose uptake following exercise may be to allow for enhanced glycogen synthesis, nutritional state can also influence the magnitude of improvements in insulin sensitivity observed following an acute bout of exercise. It has been proposed that glycogen depletion and AMPK activation may play a role in insulin sensitizing effects of exercise given AMPK γ3 KO and α2 KD mice have impaired glycogen resynthesis following exercise [4,31]. However, alternative rodent studies investigating the relationship between AMPK activation, glycogen synthesis and insulin sensitivity under different contraction (contraction length, frequency, and time) and exercise intensity (high [HIT] vs. low [LIT]) protocols have found inconsistent observations. Koshinaka et al. [143] found that whilst AMPK activation increased with exercise intensity, there were not greater improvements in insulin sensitivity when HIT was compared to LIT exercise groups. Similarly, Kim et al. [144] showed that whilst the different contraction protocols altered the level of AMPK activation, there were no differences in the level of improvement in insulin sensitivity. Furthermore, improvements in insulin sensitivity were not consistent with AMPK activation and glycogen depletion [144]. These studies suggest that whilst AMPK may be involved in insulin sensitizing effects of exercise, alternative pathways and measures other than glycogen levels and AMPK activity should be considered when determining the level of exercise-mediated improvements in insulin sensitivity.
Exercise improves insulin sensitivity and it was originally proposed that this may involve the direct interaction of AMPK with IRS1 [145]. It has been reported that AMPK rapidly phosphorylates IRS1 on S789 in vitro and treatment with AICAR increases IRS1 S789 phosphorylation in mouse C2C12 myotubes, suggesting a possible link between the potentiating effects of exercise on insulin signaling [145]. Alternative studies show that IRS1 S789 phosphorylation is negatively associated with insulin signaling [146-149]. Instead, activation of AMPK by glucose deprivation or AICAR promotes tyrosine phosphorylation of IR and IRS-1 as well as Akt T308 and S473 phosphorylation [146]. Whilst AMPK is not a tyrosine kinase, it is hypothesized that S/T phosphorylation of IR and IRS-1 by AMPK promotes allosteric activation by tyrosine kinases; however, further studies are required to confirm this [146].
An alternative mechanism that may explain the potential insulin sensitizing effects of exercise involves AMPK inhibition of the mammalian target of rapamycin/p70 ribosomal S6 kinase (mTOR/p70 S6K) signaling pathway [150]. Obese and type 2 diabetic rodents have increased mTOR/S6K signaling and this may contribute to insulin resistance [151-153]. S6K phosphorylation of IRS1 at S1101 is increased in genetic and diet-induced obese mice and has shown to induce insulin resistance [154]. Inhibition of mTOR/p70 S6K with rapamycin restores insulin sensitivity caused by amino acids in human skeletal muscle [155,156]. Endurance training has been shown to inhibit mTOR/p70 S6K signaling and reduce IRS1 serine phosphorylation in rat skeletal muscle [157], and recent studies have demonstrated that AMPK is involved in the suppression of mTOR signaling [39]. For example treatment with AICAR inhibits S6K (mTOR effector) activity in a range of mammalian cells [150]. Similarly, incubation with AICAR in C2C12 myotubes transfected with an adenovirus of a constitutively active (truncated form of AMPKα1) or KD form of AMPKα revealed that serine phosphorylation of IRS1 was decreased at a target site of mTOR/p70 S6K, which has been associated with insulin resistance [158]. Recently, AMPK was found to phosphorylate the IR in response to glucose deprivation; conditions that also suppresses mTOR activity [146]. The α2 subunit of AMPK is required for AICAR-induced inhibitory effects of mTOR signaling in skeletal muscle [159], which is consistent with alternative findings that showing that under basal conditions the α2 subunit rather than α1 subunit is required for AICAR-induced glucose uptake [26]. Thus, increased AMPK activity could be hypothesized to impair inhibitory mTOR signaling towards IRS1. Further studies are required to establish this.
The stimulation of AMPK by adiponectin may also be an alternative mechanism by which AMPK improves insulin sensitivity. Recent studies have shown that the activation of AMPK by adiponectin enhances the ability of insulin to stimulate IRS1 and Akt phosphorylation concurrently, with reduced S6K1 phosphorylation at T389 as well as IRS1 phosphorylation at S302 and S636/639 [160]. Aerobic training increases circulating adiponectin and adiponectin receptor mRNA in skeletal muscle [161], further supporting a role for AMPK signaling in improving insulin sensitivity during exercise. JNK and IKK can phosphorylate serine residues on IRS1 and impair insulin signaling; therefore, these studies may suggest that activation of AMPK during exercise may help restore insulin signaling that is impaired by these serine kinases in obesity.
The gp130 receptor IL-6 protein was one of the first identified myokines whose production in skeletal muscle and release into the circulation increases almost 100 fold in response to exercise [162]. Circulating IL-6 levels are elevated in obesity and during exercise; however, in obesity high levels of IL-6 have been linked to insulin resistance whereas during exercise IL-6 may enhance glucose uptake via AMPK and improve insulin sensitivity given IL-6 is additive to insulin in promoting glucose uptake. Carey et al. [163] was one of the first studies to show that IL-6 increases glucose disposal in humans and glucose uptake in L6 myotubes; an effect dependent on AMPK signaling. Their study and Geiger et al. [164] also suggested that IL-6 is additive to insulin in stimulating glucose uptake; however, this was not observed by all studies [165] who found that IL-6 regulated glucose uptake and glycogen synthesis via a PI3-dependent pathway and not AMPK; given human myotubes targeted for AMPK depletion by siRNA did not have impaired insulin sensitivity. In contrast, Geiger et al. [164] showed that in whole muscle, which is more biologically relevant, that only higher concentrations of IL-6 increased AMPK phosphorylation; suggesting that under physiological conditions, such as during exercise, IL-6 may play a less significant role in positively regulating metabolic processes. The following year, Nieto-Vazquez et al. [166] found that the differential effects of IL-6 may be due to the amount of exposure to the cytokine, whereby, chronic treatment induces insulin resistance via mechanisms involving protein-tyrosine phosphatase 1B, JNK activation and SOCS3-mediated inhibition of insulin signaling, while acute exposure enhances insulin-stimulated glucose uptake via the LKB1/AMPK/TBC1D4 pathway. Recently, IL-6 KO mice reveal a relatively minor role for IL-6 in promoting glucose clearance during acute treadmill exercise [167]. Postexercise IL-6 levels are positively correlated with exercise duration (reviewed in [168]), and during the recovery period it could be speculated that the effects of IL-6 on metabolism are most pronounced. The majority of the data showing a positive effect of IL-6 on substrate utilization and insulin sensitivity has relied on the infusion of supraphysiological IL-6 concentrations, which does not demonstrate the physiological role of circulating IL-6 during and following exercise. Nonetheless finding from these studies have shown that IL-6 is important for insulin secretion and glucose tolerance via glucagon like peptide 1 (GLP-1) [169]. An alternative study utilizing IL-6 KO mice has shown that IL-6 is required for improving insulin-sensitivity in high-fat fed mice following chronic (4 weeks) voluntary wheel exercise training through lowering serum retinol binding protein 4 (RBP-4) levels [167], which have been implicated in obesity-induced insulin resistance in both humans and rodents [170,171]. Therefore, whilst these studies support a role for IL-6 in improving insulin sensitivity following exercise via RBP-4 and GLP-1, the involvement in AMPK in regulating this process remains to be determined.
Whilst it has been reported that AMPK activity and protein expression may be reduced in obesity [9,172,173], chronic exercise training increases AMPK protein expression and activity [174]. Therefore, AMPK may enhance insulin sensitivity through increasing TBC1D1 and TBC1D4 phosphorylation. Consistent with this idea, studies have also shown that chronic exercise training increases both protein expression and PAS phosphorylation of TBC1D4 in both humans and rats. TBC1D4 phosphorylation (S318, 341, 588, 642, and 751) is reduced in skeletal muscle from obese type 2 diabetics under basal conditions, but can be restored to comparable levels of nondiabetics by 10 weeks of endurance training [81]. Whether similar findings are observed for TBC1D1 is currently not known. Interestingly, a two week dietary intervention to induce weight loss improved insulin sensitivity in obese humans but did not alter TBC1D1 phosphorylation. Instead, the dietary intervention did increase TBC1D1 S231 and 660 but not S700 or PAS phosphorylation in response to exercise [175].
AMPK γ3 expression is reduced following strength training in obese type 2 diabetic humans and endurance training in healthy men [10,49]. Given deletion of the γ3 isoform is associated with impaired glycogen resynthesis following exercise; it is possible that reduced γ3 expression following exercise training is an adaptive response to enhance oxidative capacity and greater preference for lipid oxidation rather than glycogen utilization. It has recently been suggested that increased basal level of AMPK activity is likely due to γ1 associated complexes given γ3 expression is reduced [176].
In conclusion, AMPK plays an important role in regulating glucose uptake during exercise and may be involved in the insulin sensitizing effects of both acute and chronic exercise. A number of possible mechanisms have been proposed to play a role in insulin sensitizing effects of exercise; however, further studies are required to fully define the role of AMPK in mediating these effects. Exercise remains as one of the most cost effective treatments providing endless health benefits including both the prevention and treatment of obesity-induced insulin resistance. Therefore, future studies aimed at identifying the underlying mechanisms involved in the insulin sensitizing effects of exercise are essential for the development of new strategies both pharmacological and nonpharmacological such as exercise that target these diseases.
Figures and Tables
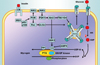 | Fig. 1Regulation of insulin-stimulated glucose uptake and glycogen synthesis. Insulin stimulates glucose uptake by binding to the insulin receptor (IR), this promotes autophosphorylation and subsequent activation of insulin receptor substrate 1 (IRS1) and PI3 kinase via SH2 interaction with regulatory p85 and catalytic p110 subunits. This promotes association with phosphatidylinositol 4,5-bisphosphate (PIP2) at the plasma membrane, which is converted to phosphatidylinositol 3,4,5-triphosphate (PIP3), which induces a conformational change in Akt that allows Akt phosphorylation and subsequent phosphorylation and inhibition of the Rab-GAP activating protein tre-2/USP6, BUB2, cdc16 domain family member 4 (TBC1D4). Rac/actin can also promote glucose uptake by promoting actin remodeling. Once glucose enters the cell it can be metabolized through glycolysis to produce ATP or utilized for glycogen synthesis. Glycogen synthesis involves phosphorylation and inhibition of glycogen synthase kinase 3 (GSK3) by Akt, which activates glycogen synthase (GS); promoting the conversion of glucose-6 phosphate (G6P) to G1P then uridine diphosphoglucose (UDP-G), which is targeted towards glycogen. AMPK can phosphorylate and inhibit GS; however, G6P can override this inhibitory effect. PTG, protein targeting to glycogen. |
 | Fig. 2Regulation of glucose uptake during exercise and muscle contractions. During contraction, there is depolarization of T-tubules (plasma membrane only found in skeletal muscle) that causes calcium (Ca2+) release from the sarcoplasmic reticulum, which triggers actin and myosin interaction (red; thick myosin and thin actin filaments). The energy demand of contraction increases the ratio of adenosine monophosphate (AMP)/adenosine triphosphate (ATP), which stimulates AMP-associated protein kinase (AMPK). Both tre-2/USP6, BUB2, cdc16 domain family member 4 and 1 (TBC1D 4 and 1) are involved in regulating glucose uptake in response to contraction; however, it has recently been discovered that TBC1D1 plays a more pivotal role. AMPK can phosphorylate both TBC1D4 and TBC1D1; however, recent studies have shown that during contraction there is a strong correlation between AMPK phosphorylation of TBC1D1 and 14-3-3 binding (proteins that are proposed to be important for regulation of GAP function of TBC1D1 upon phosphorylation), which allows dissociation of Rab proteins and glucose transporter 4 (GLUT4) translocation to the plasma membrane and glucose uptake. AK, adenylate kinase, the enzyme required for generation of AMP. |
ACKNOWLEDGMENTS
I would like to thank Dr. Gregory R. Steinberg for insightful suggestions on this manuscript.
References
1. Dyck JR, Gao G, Widmer J, Stapleton D, Fernandez CS, Kemp BE, Witters LA. Regulation of 5'-AMP-activated protein kinase activity by the noncatalytic beta and gamma subunits. J Biol Chem. 1996. 271:17798–17803.
2. Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J. 2000. 346 Pt 3:659–669.
3. Luptak I, Shen M, He H, Hirshman MF, Musi N, Goodyear LJ, Yan J, Wakimoto H, Morita H, Arad M, Seidman CE, Seidman JG, Ingwall JS, Balschi JA, Tian R. Aberrant activation of AMP-activated protein kinase remodels metabolic network in favor of cardiac glycogen storage. J Clin Invest. 2007. 117:1432–1439.
4. Barnes BR, Marklund S, Steiler TL, Walter M, Hjalm G, Amarger V, Mahlapuu M, Leng Y, Johansson C, Galuska D, Lindgren K, Abrink M, Stapleton D, Zierath JR, Andersson L. The 5'-AMP-activated protein kinase gamma3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J Biol Chem. 2004. 279:38441–38447.
5. Mahlapuu M, Johansson C, Lindgren K, Hjalm G, Barnes BR, Krook A, Zierath JR, Andersson L, Marklund S. Expression profiling of the gamma-subunit isoforms of AMP-activated protein kinase suggests a major role for gamma3 in white skeletal muscle. Am J Physiol Endocrinol Metab. 2004. 286:E194–E200.
6. Yu H, Fujii N, Hirshman MF, Pomerleau JM, Goodyear LJ. Cloning and characterization of mouse 5'-AMP-activated protein kinase gamma3 subunit. Am J Physiol Cell Physiol. 2004. 286:C283–C292.
7. Chen Z, Heierhorst J, Mann RJ, Mitchelhill KI, Michell BJ, Witters LA, Lynch GS, Kemp BE, Stapleton D. Expression of the AMP-activated protein kinase beta1 and beta2 subunits in skeletal muscle. FEBS Lett. 1999. 460:343–348.
8. Murphy RM. Enhanced technique to measure proteins in single segments of human skeletal muscle fibers: fiber-type dependence of AMPK-alpha1 and -beta1. J Appl Physiol. 2011. 110:820–825.
9. Mortensen B, Poulsen P, Wegner L, Stender-Petersen KL, Ribel-Madsen R, Friedrichsen M, Birk JB, Vaag A, Wojtaszewski JF. Genetic and metabolic effects on skeletal muscle AMPK in young and older twins. Am J Physiol Endocrinol Metab. 2009. 297:E956–E964.
10. Wojtaszewski JF, Birk JB, Frosig C, Holten M, Pilegaard H, Dela F. 5'AMP activated protein kinase expression in human skeletal muscle: effects of strength training and type 2 diabetes. J Physiol. 2005. 564(Pt 2):563–573.
11. Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007. 403:139–148.
12. Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, Kemp BE. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011. 332:1433–1435.
13. Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011. 472:230–233.
14. Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996. 271:27879–27887.
15. Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003. 2:28.
16. Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003. 13:2004–2008.
17. Koh HJ, Arnolds DE, Fujii N, Tran TT, Rogers MJ, Jessen N, Li Y, Liew CW, Ho RC, Hirshman MF, Kulkarni RN, Kahn CR, Goodyear LJ. Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3. Mol Cell Biol. 2006. 26:8217–8227.
18. Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005. 24:1810–1820.
19. Thomson DM, Porter BB, Tall JH, Kim HJ, Barrow JR, Winder WW. Skeletal muscle and heart LKB1 deficiency causes decreased voluntary running and reduced muscle mitochondrial marker enzyme expression in mice. Am J Physiol Endocrinol Metab. 2007. 292:E196–E202.
20. Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005. 280:29060–29066.
21. Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005. 2:9–19.
22. Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005. 2:21–33.
23. DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009. 32:Suppl 2. S157–S163.
24. DeFronzo RA, Ferrannini E, Sato Y, Felig P, Wahren J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest. 1981. 68:1468–1474.
25. Thiebaud D, Jacot E, DeFronzo RA, Maeder E, Jequier E, Felber JP. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982. 31:957–963.
26. Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not alpha1 5'-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004. 279:1070–1079.
27. Dzamko N, van Denderen BJ, Hevener AL, Jorgensen SB, Honeyman J, Galic S, Chen ZP, Watt MJ, Campbell DJ, Steinberg GR, Kemp BE. AMPK beta1 deletion reduces appetite, preventing obesity and hepatic insulin resistance. J Biol Chem. 2010. 285:115–122.
28. Steinberg GR, O'Neill HM, Dzamko NL, Galic S, Naim T, Koopman R, Jorgensen SB, Honeyman J, Hewitt K, Chen ZP, Schertzer JD, Scott JW, Koentgen F, Lynch GS, Watt MJ, van Denderen BJ, Campbell DJ, Kemp BE. Whole body deletion of AMP-activated protein kinase {beta}2 reduces muscle AMPK activity and exercise capacity. J Biol Chem. 2010. 285:37198–37209.
29. Dasgupta B, Ju JS, Sasaki Y, Liu X, Jung SR, Higashida K, Lindquist D, Milbrandt J. The AMPK beta2 subunit is required for energy homeostasis during metabolic stress. Mol Cell Biol. 2012. 32:2837–2848.
30. O'Neill HM, Maarbjerg SJ, Crane JD, Jeppesen J, Jorgensen SB, Schertzer JD, Shyroka O, Kiens B, van Denderen BJ, Tarnopolsky MA, Kemp BE, Richter EA, Steinberg GR. AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc Natl Acad Sci U S A. 2011. 108:16092–16097.
31. Mu J, Brozinick JT Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001. 7:1085–1094.
32. Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, Goodyear LJ. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem. 2005. 280:39033–39041.
33. Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003. 111:91–98.
34. Dzamko N, Schertzer JD, Ryall JG, Steel R, Macaulay SL, Wee S, Chen ZP, Michell BJ, Oakhill JS, Watt MJ, Jorgensen SB, Lynch GS, Kemp BE, Steinberg GR. AMPK-independent pathways regulate skeletal muscle fatty acid oxidation. J Physiol. 2008. 586:5819–5831.
35. Viollet B, Andreelli F, Jorgensen SB, Perrin C, Flamez D, Mu J, Wojtaszewski JF, Schuit FC, Birnbaum M, Richter E, Burcelin R, Vaulont S. Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem Soc Trans. 2003. 31:216–219.
36. Beck Jorgensen S, O'Neill HM, Hewitt K, Kemp BE, Steinberg GR. Reduced AMP-activated protein kinase activity in mouse skeletal muscle does not exacerbate the development of insulin resistance with obesity. Diabetologia. 2009. 52:2395–2404.
37. Fujii N, Ho RC, Manabe Y, Jessen N, Toyoda T, Holland WL, Summers SA, Hirshman MF, Goodyear LJ. Ablation of AMP-activated protein kinase alpha2 activity exacerbates insulin resistance induced by high-fat feeding of mice. Diabetes. 2008. 57:2958–2966.
38. Quinn JM, Tam S, Sims NA, Saleh H, McGregor NE, Poulton IJ, Scott JW, Gillespie MT, Kemp BE, van Denderen BJ. Germline deletion of AMP-activated protein kinase beta subunits reduces bone mass without altering osteoclast differentiation or function. FASEB J. 2010. 24:275–285.
39. Egan D, Kim J, Shaw RJ, Guan KL. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy. 2011. 7:643–644.
40. Nilsson EC, Long YC, Martinsson S, Glund S, Garcia-Roves P, Svensson LT, Andersson L, Zierath JR, Mahlapuu M. Opposite transcriptional regulation in skeletal muscle of AMP-activated protein kinase gamma3 R225Q transgenic versus knock-out mice. J Biol Chem. 2006. 281:7244–7252.
41. Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010. 11:213–219.
42. Costford SR, Kavaslar N, Ahituv N, Chaudhry SN, Schackwitz WS, Dent R, Pennacchio LA, McPherson R, Harper ME. Gain-of-function R225W mutation in human AMPKgamma(3) causing increased glycogen and decreased triglyceride in skeletal muscle. PLoS One. 2007. 2:e903.
43. Crawford SA, Costford SR, Aguer C, Thomas SC, deKemp RA, DaSilva JN, Lafontaine D, Kendall M, Dent R, Beanlands RS, McPherson R, Harper ME. Naturally occurring R225W mutation of the gene encoding AMP-activated protein kinase (AMPK)gamma(3) results in increased oxidative capacity and glucose uptake in human primary myotubes. Diabetologia. 2010. 53:1986–1997.
44. Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, McGarry K, Seidman JG, Seidman CE. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002. 109:357–362.
45. Milan D, Jeon JT, Looft C, Amarger V, Robic A, Thelander M, Rogel-Gaillard C, Paul S, Iannuccelli N, Rask L, Ronne H, Lundstrom K, Reinsch N, Gellin J, Kalm E, Roy PL, Chardon P, Andersson L. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science. 2000. 288:1248–1251.
46. Gollob MH, Seger JJ, Gollob TN, Tapscott T, Gonzales O, Bachinski L, Roberts R. Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy. Circulation. 2001. 104:3030–3033.
47. Gollob MH, Green MS, Tang AS, Gollob T, Karibe A, Ali Hassan AS, Ahmad F, Lozado R, Shah G, Fananapazir L, Bachinski LL, Roberts R. Identification of a gene responsible for familial Wolff-Parkinson-White syndrome. N Engl J Med. 2001. 344:1823–1831.
48. Barnes BR, Glund S, Long YC, Hjalm G, Andersson L, Zierath JR. 5'-AMP-activated protein kinase regulates skeletal muscle glycogen content and ergogenics. FASEB J. 2005. 19:773–779.
49. Frosig C, Jorgensen SB, Hardie DG, Richter EA, Wojtaszewski JF. 5'-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004. 286:E411–E417.
50. Sahlin K, Tonkonogi M, Soderlund K. Energy supply and muscle fatigue in humans. Acta Physiol Scand. 1998. 162:261–266.
51. Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5' AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998. 47:1369–1373.
52. Hutber CA, Hardie DG, Winder WW. Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Am J Physiol. 1997. 272(2 Pt 1):E262–E266.
53. Ihlemann J, Ploug T, Hellsten Y, Galbo H. Effect of tension on contraction-induced glucose transport in rat skeletal muscle. Am J Physiol. 1999. 277(2 Pt 1):E208–E214.
54. Rasmussen BB, Hancock CR, Winder WW. Postexercise recovery of skeletal muscle malonyl-CoA, acetyl-CoA carboxylase, and AMP-activated protein kinase. J Appl Physiol. 1998. 85:1629–1634.
55. Rasmussen BB, Winder WW. Effect of exercise intensity on skeletal muscle malonyl-CoA and acetyl-CoA carboxylase. J Appl Physiol. 1997. 83:1104–1109.
56. Vavvas D, Apazidis A, Saha AK, Gamble J, Patel A, Kemp BE, Witters LA, Ruderman NB. Contraction-induced changes in acetyl-CoA carboxylase and 5'-AMP-activated kinase in skeletal muscle. J Biol Chem. 1997. 272:13255–13261.
57. Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5'-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000. 528 Pt 1:221–226.
58. Chen ZP, Stephens TJ, Murthy S, Canny BJ, Hargreaves M, Witters LA, Kemp BE, McConell GK. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes. 2003. 52:2205–2212.
59. Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5'AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000. 273:1150–1155.
60. Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996. 270(2 Pt 1):E299–E304.
61. Winder WW, Wilson HA, Hardie DG, Rasmussen BB, Hutber CA, Call GB, Clayton RD, Conley LM, Yoon S, Zhou B. Phosphorylation of rat muscle acetyl-CoA carboxylase by AMP-activated protein kinase and protein kinase A. J Appl Physiol. 1997. 82:219–225.
62. Chen TC, Hsieh SS. The effects of repeated maximal voluntary isokinetic eccentric exercise on recovery from muscle damage. Res Q Exerc Sport. 2000. 71:260–266.
63. Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000. 49:527–531.
64. Kennedy JW, Hirshman MF, Gervino EV, Ocel JV, Forse RA, Hoenig SJ, Aronson D, Goodyear LJ, Horton ES. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes. 1999. 48:1192–1197.
65. Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996. 15:6541–6551.
66. Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997. 7:261–269.
67. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005. 307:1098–1101.
68. Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem. 2006. 281:31478–31485.
69. Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003. 278:14599–14602.
70. Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J. 2008. 409:449–459.
71. Ishikura S, Klip A. Muscle cells engage Rab8A and myosin Vb in insulin-dependent GLUT4 translocation. Am J Physiol Cell Physiol. 2008. 295:C1016–C1025.
72. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001. 414:799–806.
73. Cartee GD, Funai K. Exercise and insulin: convergence or divergence at AS160 and TBC1D1? Exerc Sport Sci Rev. 2009. 37:188–195.
74. Pehmoller C, Treebak JT, Birk JB, Chen S, Mackintosh C, Hardie DG, Richter EA, Wojtaszewski JF. Genetic disruption of AMPK signaling abolishes both contraction- and insulin-stimulated TBC1D1 phosphorylation and 14-3-3 binding in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2009. 297:E665–E675.
75. Chen S, Wasserman DH, MacKintosh C, Sakamoto K. Mice with AS160/TBC1D4-Thr649Ala knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab. 2011. 13:68–79.
76. Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 2006. 55:2067–2076.
77. Treebak JT, Frosig C, Pehmoller C, Chen S, Maarbjerg SJ, Brandt N, MacKintosh C, Zierath JR, Hardie DG, Kiens B, Richter EA, Pilegaard H, Wojtaszewski JF. Potential role of TBC1D4 in enhanced post-exercise insulin action in human skeletal muscle. Diabetologia. 2009. 52:891–900.
78. Ducommun S, Wang HY, Sakamoto K, Mackintosh C, Chen S. Thr649Ala-AS160 knock-in mutation does not impair contraction/AICAR-induced glucose transport in mouse muscle. Am J Physiol Endocrinol Metab. 2012. 302:E1036–E1043.
79. Chen S, Mackintosh C. Differential regulation of NHE1 phosphorylation and glucose uptake by inhibitors of the ERK pathway and p90RSK in 3T3-L1 adipocytes. Cell Signal. 2009. 21:1984–1993.
80. Geraghty KM, Chen S, Harthill JE, Ibrahim AF, Toth R, Morrice NA, Vandermoere F, Moorhead GB, Hardie DG, MacKintosh C. Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem J. 2007. 407:231–241.
81. Vind BF, Pehmoller C, Treebak JT, Birk JB, Hey-Mogensen M, Beck-Nielsen H, Zierath JR, Wojtaszewski JF, Hojlund K. Impaired insulin-induced site-specific phosphorylation of TBC1 domain family, member 4 (TBC1D4) in skeletal muscle of type 2 diabetes patients is restored by endurance exercise-training. Diabetologia. 2011. 54:157–167.
82. Bouzakri K, Zachrisson A, Al-Khalili L, Zhang BB, Koistinen HA, Krook A, Zierath JR. siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab. 2006. 4:89–96.
83. Hoehn KL, Hohnen-Behrens C, Cederberg A, Wu LE, Turner N, Yuasa T, Ebina Y, James DE. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 2008. 7:421–433.
84. Shirakami A, Toyonaga T, Tsuruzoe K, Shirotani T, Matsumoto K, Yoshizato K, Kawashima J, Hirashima Y, Miyamura N, Kahn CR, Araki E. Heterozygous knockout of the IRS-1 gene in mice enhances obesity-linked insulin resistance: a possible model for the development of type 2 diabetes. J Endocrinol. 2002. 174:309–319.
85. Cleasby ME, Reinten TA, Cooney GJ, James DE, Kraegen EW. Functional studies of Akt isoform specificity in skeletal muscle in vivo: maintained insulin sensitivity despite reduced insulin receptor substrate-1 expression. Mol Endocrinol. 2007. 21:215–228.
86. Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell. 2006. 17:4484–4493.
87. Zaid H, Antonescu CN, Randhawa VK, Klip A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem J. 2008. 413:201–215.
88. JeBailey L, Rudich A, Huang X, Di Ciano-Oliveira C, Kapus A, Klip A. Skeletal muscle cells and adipocytes differ in their reliance on TC10 and Rac for insulin-induced actin remodeling. Mol Endocrinol. 2004. 18:359–372.
89. JeBailey L, Wanono O, Niu W, Roessler J, Rudich A, Klip A. Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells. Diabetes. 2007. 56:394–403.
90. Tsakiridis T, Vranic M, Klip A. Disassembly of the actin network inhibits insulin-dependent stimulation of glucose transport and prevents recruitment of glucose transporters to the plasma membrane. J Biol Chem. 1994. 269:29934–29942.
91. Wang Q, Somwar R, Bilan PJ, Liu Z, Jin J, Woodgett JR, Klip A. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol. 1999. 19:4008–4018.
92. Uberall F, Hellbert K, Kampfer S, Maly K, Villunger A, Spitaler M, Mwanjewe J, Baier-Bitterlich G, Baier G, Grunicke HH. Evidence that atypical protein kinase C-lambda and atypical protein kinase C-zeta participate in Ras-mediated reorganization of the F-actin cytoskeleton. J Cell Biol. 1999. 144:413–425.
93. Stone S, Abkevich V, Russell DL, Riley R, Timms K, Tran T, Trem D, Frank D, Jammulapati S, Neff CD, Iliev D, Gress R, He G, Frech GC, Adams TD, Skolnick MH, Lanchbury JS, Gutin A, Hunt SC, Shattuck D. TBC1D1 is a candidate for a severe obesity gene and evidence for a gene/gene interaction in obesity predisposition. Hum Mol Genet. 2006. 15:2709–2720.
94. Meyre D, Farge M, Lecoeur C, Proenca C, Durand E, Allegaert F, Tichet J, Marre M, Balkau B, Weill J, Delplanque J, Froguel P. R125W coding variant in TBC1D1 confers risk for familial obesity and contributes to linkage on chromosome 4p14 in the French population. Hum Mol Genet. 2008. 17:1798–1802.
95. An D, Toyoda T, Taylor EB, Yu H, Fujii N, Hirshman MF, Goodyear LJ. TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle. Diabetes. 2010. 59:1358–1365.
96. Roach WG, Chavez JA, Miinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J. 2007. 403:353–358.
97. Chadt A, Leicht K, Deshmukh A, Jiang LQ, Scherneck S, Bernhardt U, Dreja T, Vogel H, Schmolz K, Kluge R, Zierath JR, Hultschig C, Hoeben RC, Schurmann A, Joost HG, Al-Hasani H. Tbc1d1 mutation in lean mouse strain confers leanness and protects from diet-induced obesity. Nat Genet. 2008. 40:1354–1359.
98. Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem. 2008. 283:9787–9796.
99. Peck GR, Chavez JA, Roach WG, Budnik BA, Lane WS, Karlsson HK, Zierath JR, Lienhard GE. Insulin-stimulated phosphorylation of the Rab GTPase-activating protein TBC1D1 regulates GLUT4 translocation. J Biol Chem. 2009. 284:30016–30023.
100. Barnes BR, Zierath JR. Role of AMP: activated protein kinase in the control of glucose homeostasis. Curr Mol Med. 2005. 5:341–348.
101. Funai K, Cartee GD. Inhibition of contraction-stimulated AMP-activated protein kinase inhibits contraction-stimulated increases in PAS-TBC1D1 and glucose transport without altering PAS-AS160 in rat skeletal muscle. Diabetes. 2009. 58:1096–1104.
102. Vichaiwong K, Purohit S, An D, Toyoda T, Jessen N, Hirshman MF, Goodyear LJ. Contraction regulates site-specific phosphorylation of TBC1D1 in skeletal muscle. Biochem J. 2010. 431:311–320.
103. Yeh JI, Gulve EA, Rameh L, Birnbaum MJ. The effects of wortmannin on rat skeletal muscle. Dissociation of signaling pathways for insulin- and contraction-activated hexose transport. J Biol Chem. 1995. 270:2107–2111.
104. Whitehead JP, Soos MA, Aslesen R, O'Rahilly S, Jensen J. Contraction inhibits insulin-stimulated insulin receptor substrate-1/2-associated phosphoinositide 3-kinase activity, but not protein kinase B activation or glucose uptake, in rat muscle. Biochem J. 2000. 349(Pt 3):775–781.
105. Lund S, Holman GD, Schmitz O, Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci U S A. 1995. 92:5817–5821.
106. Brozinick JT Jr, Birnbaum MJ. Insulin, but not contraction, activates Akt/PKB in isolated rat skeletal muscle. J Biol Chem. 1998. 273:14679–14682.
107. Dumke CL, Wetter AC, Arias EB, Kahn CR, Cartee GD. Absence of insulin receptor substrate-1 expression does not alter GLUT1 or GLUT4 abundance or contraction-stimulated glucose uptake by mouse skeletal muscle. Horm Metab Res. 2001. 33:696–700.
108. Sakamoto K, Arnolds DE, Fujii N, Kramer HF, Hirshman MF, Goodyear LJ. Role of Akt2 in contraction-stimulated cell signaling and glucose uptake in skeletal muscle. Am J Physiol Endocrinol Metab. 2006. 291:E1031–E1037.
109. Constable SH, Favier RJ, Cartee GD, Young DA, Holloszy JO. Muscle glucose transport: interactions of in vitro contractions, insulin, and exercise. J Appl Physiol. 1988. 64:2329–2332.
110. Gao J, Ren J, Gulve EA, Holloszy JO. Additive effect of contractions and insulin on GLUT-4 translocation into the sarcolemma. J Appl Physiol. 1994. 77:1597–1601.
111. Martin IK, Katz A, Wahren J. Splanchnic and muscle metabolism during exercise in NIDDM patients. Am J Physiol. 1995. 269(3 Pt 1):E583–E590.
112. Han X, Ploug T, Galbo H. Effect of diet on insulin- and contraction-mediated glucose transport and uptake in rat muscle. Am J Physiol. 1995. 269(3 Pt 2):R544–R551.
113. King PA, Betts JJ, Horton ED, Horton ES. Exercise, unlike insulin, promotes glucose transporter translocation in obese Zucker rat muscle. Am J Physiol. 1993. 265(2 Pt 2):R447–R452.
114. Brozinick JT Jr, Etgen GJ Jr, Yaspelkis BB 3rd, Ivy JL. Contraction-activated glucose uptake is normal in insulin-resistant muscle of the obese Zucker rat. J Appl Physiol. 1992. 73:382–387.
115. Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes. 2005. 54:41–50.
116. Kramer HF, Taylor EB, Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Calmodulin-binding domain of AS160 regulates contraction- but not insulin-stimulated glucose uptake in skeletal muscle. Diabetes. 2007. 56:2854–2862.
117. Frosig C, Pehmoller C, Birk JB, Richter EA, Wojtaszewski JF. Exercise-induced TBC1D1 Ser237 phosphorylation and 14-3-3 protein binding capacity in human skeletal muscle. J Physiol. 2010. 588(Pt 22):4539–4548.
118. Lee-Young RS, Griffee SR, Lynes SE, Bracy DP, Ayala JE, McGuinness OP, Wasserman DH. Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J Biol Chem. 2009. 284:23925–23934.
119. Lefort N, St-Amand E, Morasse S, Cote CH, Marette A. The alpha-subunit of AMPK is essential for submaximal contraction-mediated glucose transport in skeletal muscle in vitro. Am J Physiol Endocrinol Metab. 2008. 295:E1447–E1454.
120. Merry TL, Steinberg GR, Lynch GS, McConell GK. Skeletal muscle glucose uptake during contraction is regulated by nitric oxide and ROS independently of AMPK. Am J Physiol Endocrinol Metab. 2010. 298:E577–E585.
121. Koh HJ, Toyoda T, Fujii N, Jung MM, Rathod A, Middelbeek RJ, Lessard SJ, Treebak JT, Tsuchihara K, Esumi H, Richter EA, Wojtaszewski JF, Hirshman MF, Goodyear LJ. Sucrose nonfermenting AMPK-related kinase (SNARK) mediates contraction-stimulated glucose transport in mouse skeletal muscle. Proc Natl Acad Sci U S A. 2010. 107:15541–15546.
122. Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2007. 292:E1191–E1200.
123. Funai K, Schweitzer GG, Castorena CM, Kanzaki M, Cartee GD. In vivo exercise followed by in vitro contraction additively elevates subsequent insulin-stimulated glucose transport by rat skeletal muscle. Am J Physiol Endocrinol Metab. 2010. 298:E999–1010.
124. Howlett KF, Mathews A, Garnham A, Sakamoto K. The effect of exercise and insulin on AS160 phosphorylation and 14-3-3 binding capacity in human skeletal muscle. Am J Physiol Endocrinol Metab. 2008. 294:E401–E407.
125. Aschenbach WG, Suzuki Y, Breeden K, Prats C, Hirshman MF, Dufresne SD, Sakamoto K, Vilardo PG, Steele M, Kim JH, Jing SL, Goodyear LJ, DePaoli-Roach AA. The muscle-specific protein phosphatase PP1G/R(GL)(G(M))is essential for activation of glycogen synthase by exercise. J Biol Chem. 2001. 276:39959–39967.
126. Jensen J, Lai YC. Regulation of muscle glycogen synthase phosphorylation and kinetic properties by insulin, exercise, adrenaline and role in insulin resistance. Arch Physiol Biochem. 2009. 115:13–21.
127. Bouskila M, Hunter RW, Ibrahim AF, Delattre L, Peggie M, van Diepen JA, Voshol PJ, Jensen J, Sakamoto K. Allosteric regulation of glycogen synthase controls glycogen synthesis in muscle. Cell Metab. 2010. 12:456–466.
128. McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005. 24:1571–1583.
129. Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, Kemp BE, Stapleton D. AMPK beta subunit targets metabolic stress sensing to glycogen. Curr Biol. 2003. 13:867–871.
130. McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009. 9:23–34.
131. Bendayan M, Londono I, Kemp BE, Hardie GD, Ruderman N, Prentki M. Association of AMP-activated protein kinase subunits with glycogen particles as revealed in situ by immunoelectron microscopy. J Histochem Cytochem. 2009. 57:963–971.
132. Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta. 1989. 1012:81–86.
133. Miyamoto L, Toyoda T, Hayashi T, Yonemitsu S, Nakano M, Tanaka S, Ebihara K, Masuzaki H, Hosoda K, Ogawa Y, Inoue G, Fushiki T, Nakao K. Effect of acute activation of 5'-AMP-activated protein kinase on glycogen regulation in isolated rat skeletal muscle. J Appl Physiol. 2007. 102:1007–1013.
134. Wojtaszewski JF, Jorgensen SB, Hellsten Y, Hardie DG, Richter EA. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes. 2002. 51:284–292.
135. Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, Richter EA, Wojtaszewski JF. The alpha2-5'AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes. 2004. 53:3074–3081.
136. Bultot L, Guigas B, Von Wilamowitz-Moellendorff A, Maisin L, Vertommen D, Hussain N, Beullens M, Guinovart JJ, Foretz M, Viollet B, Sakamoto K, Hue L, Rider MH. AMP-activated protein kinase phosphorylates and inactivates liver glycogen synthase. Biochem J. 2012. 443:193–203.
137. Hunter RW, Treebak JT, Wojtaszewski JF, Sakamoto K. Molecular mechanism by which AMP-activated protein kinase activation promotes glycogen accumulation in muscle. Diabetes. 2011. 60:766–774.
138. Jorgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005. 19:1146–1148.
139. Steinberg GR, O'Neill HM, Dzamko NL, Galic S, Naim T, Koopman R, Jorgensen SB, Honeyman J, Hewitt K, Chen ZP, Schertzer JD, Scott JW, Koentgen F, Lynch GS, Watt MJ, van Denderen BJ, Campbell DJ, Kemp BE. Whole body deletion of AMP-activated protein kinase {beta}2 reduces muscle AMPK activity and exercise capacity. J Biol Chem. 2010. 285:37198–37209.
140. Mu J, Barton ER, Birnbaum MJ. Selective suppression of AMP-activated protein kinase in skeletal muscle: update on 'lazy mice'. Biochem Soc Trans. 2003. 31(Pt 1):236–241.
141. Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990. 322:223–228.
142. Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2009. 297:E242–E251.
143. Koshinaka K, Sano A, Howlett KF, Yamazaki T, Sasaki M, Sakamoto K, Kawanaka K. Effect of high-intensity intermittent swimming on postexercise insulin sensitivity in rat epitrochlearis muscle. Metabolism. 2008. 57:749–756.
144. Kim J, Solis RS, Arias EB, Cartee GD. Postcontraction insulin sensitivity: relationship with contraction protocol, glycogen concentration, and 5' AMP-activated protein kinase phosphorylation. J Appl Physiol. 2004. 96:575–583.
145. Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5'-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem. 2001. 276:46912–46916.
146. Chopra I, Li HF, Wang H, Webster KA. Phosphorylation of the insulin receptor by AMP-activated protein kinase (AMPK) promotes ligand-independent activation of the insulin signalling pathway in rodent muscle. Diabetologia. 2012. 55:783–794.
147. Zakikhani M, Blouin MJ, Piura E, Pollak MN. Metformin and rapamycin have distinct effects on the AKT pathway and proliferation in breast cancer cells. Breast Cancer Res Treat. 2010. 123:271–279.
148. Horike N, Takemori H, Katoh Y, Doi J, Min L, Asano T, Sun XJ, Yamamoto H, Kasayama S, Muraoka M, Nonaka Y, Okamoto M. Adipose-specific expression, phosphorylation of Ser794 in insulin receptor substrate-1, and activation in diabetic animals of salt-inducible kinase-2. J Biol Chem. 2003. 278:18440–18447.
149. Qiao LY, Zhande R, Jetton TL, Zhou G, Sun XJ. In vivo phosphorylation of insulin receptor substrate 1 at serine 789 by a novel serine kinase in insulin-resistant rodents. J Biol Chem. 2002. 277:26530–26539.
150. Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, Kemp BE, Witters LA, Mimura O, Yonezawa K. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells. 2003. 8:65–79.
151. Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004. 431:200–205.
152. Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005. 146:1473–1481.
153. Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007. 13:252–259.
154. Tremblay F, Brule S, Hee Um S, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, Marette A. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient-and obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2007. 104:14056–14061.
155. Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, Roth E, Furnsinn C, Promintzer M, Anderwald C, Bischof M, Roden M. The mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007. 56:1600–1607.
156. Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, Nowotny P, Waldhausl W, Marette A, Roden M. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005. 54:2674–2684.
157. Glynn EL, Lujan HL, Kramer VJ, Drummond MJ, DiCarlo SE, Rasmussen BB. A chronic increase in physical activity inhibits fed-state mTOR/S6K1 signaling and reduces IRS-1 serine phosphorylation in rat skeletal muscle. Appl Physiol Nutr Metab. 2008. 33:93–101.
158. Ju JS, Gitcho MA, Casmaer CA, Patil PB, Han DG, Spencer SA, Fisher JS. Potentiation of insulin-stimulated glucose transport by the AMP-activated protein kinase. Am J Physiol Cell Physiol. 2007. 292:C564–C572.
159. Deshmukh AS, Treebak JT, Long YC, Viollet B, Wojtaszewski JF, Zierath JR. Role of adenosine 5'-monophosphate-activated protein kinase subunits in skeletal muscle mammalian target of rapamycin signaling. Mol Endocrinol. 2008. 22:1105–1112.
160. Wang C, Mao X, Wang L, Liu M, Wetzel MD, Guan KL, Dong LQ, Liu F. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J Biol Chem. 2007. 282:7991–7996.
161. Bluher M, Bullen JW Jr, Lee JH, Kralisch S, Fasshauer M, Kloting N, Niebauer J, Schon MR, Williams CJ, Mantzoros CS. Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: associations with metabolic parameters and insulin resistance and regulation by physical training. J Clin Endocrinol Metab. 2006. 91:2310–2316.
162. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008. 88:1379–1406.
163. Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006. 55:2688–2697.
164. Geiger PC, Hancock C, Wright DC, Han DH, Holloszy JO. IL-6 increases muscle insulin sensitivity only at superphysiological levels. Am J Physiol Endocrinol Metab. 2007. 292:E1842–E1846.
165. Al-Khalili L, Bouzakri K, Glund S, Lonnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol. 2006. 20:3364–3375.
166. Nieto-Vazquez I, Fernandez-Veledo S, de Alvaro C, Lorenzo M. Dual role of interleukin-6 in regulating insulin sensitivity in murine skeletal muscle. Diabetes. 2008. 57:3211–3221.
167. Benrick A, Wallenius V, Asterholm IW. Interleukin-6 mediates exercise-induced increase in insulin sensitivity in mice. Exp Physiol. 2012. 97:1224–1235.
168. Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006. 12:6–33.
169. Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD, Hansen AM, Reinecke M, Konrad D, Gassmann M, Reimann F, Halban PA, Gromada J, Drucker DJ, Gribble FM, Ehses JA, Donath MY. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011. 17:1481–1489.
170. Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006. 354:2552–2563.
171. Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005. 436:356–362.
172. Lee-Young RS, Ayala JE, Fueger PT, Mayes WH, Kang L, Wasserman DH. Obesity impairs skeletal muscle AMPK signaling during exercise: role of AMPKalpha2 in the regulation of exercise capacity in vivo. Int J Obes (Lond). 2011. 35:982–989.
173. Steinberg GR, Smith AC, Van Denderen BJ, Chen Z, Murthy S, Campbell DJ, Heigenhauser GJ, Dyck DJ, Kemp BE. AMP-activated protein kinase is not down-regulated in human skeletal muscle of obese females. J Clin Endocrinol Metab. 2004. 89:4575–4580.
174. Lee-Young RS, Canny BJ, Myers DE, McConell GK. AMPK activation is fiber type specific in human skeletal muscle: effects of exercise and short-term exercise training. J Appl Physiol. 2009. 107:283–289.
175. Jessen N, An D, Lihn AS, Nygren J, Hirshman MF, Thorell A, Goodyear LJ. Exercise increases TBC1D1 phosphorylation in human skeletal muscle. Am J Physiol Endocrinol Metab. 2011. 301:E164–E171.
176. Friedrichsen M, Mortensen B, Pehmøller C, Birk JB, Wojtaszewski JF. Exercise-induced AMPK activity in skeletal muscle: role in glucose uptake and insulin sensitivity. Mol Cell Endocrinol. Epub 2012 Jul 11. DOI: http://dx.doi.org/10.1016/j.mce.2012.06.013.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download