Abstract
Background
Sleep disturbances are common in individuals with diabetes. Patients with diabetes have higher rates of insomnia, excessive daytime sleepiness and increased incidence of restless leg syndrome. The purpose of our study was to investigate the prevalence and determine the predictors of sleep dysfunction in patients with type 2 diabetes in a southeast Asian Indian population.
Methods
We enrolled 120 patients with type 2 diabetes who attended an endocrinology clinic in a tertiary-care hospital. After we collected their demographic data, we recorded their anthropometric measurements. Fasting, postprandial blood glucose values and glycosylated hemoglobin (HbA1c) values were then obtained. Quality of sleep was evaluated in all the patients through the Pittsburgh Sleep Quality Index (PSQI), which is a questionnaire that assesses sleep quality and disturbances over a monthlong period. A Global Sleep Quality score ≥5 discriminates between good and poor sleepers.
Results
The mean global PSQI score was 7.08 (standard deviation, 3.89), which suggested poor sleep quality in this population. Sixty-nine percent of patients had a global PSQI score ≥5, indicating that they were "poor sleepers." The global PSQI score positively correlated with the duration of diabetes and was also independent of other variables such as age, gender, body mass index, HbA1c, or medications.
Conclusion
We found a high prevalence of sleep dysfunction in patients with type 2 diabetes. We also found a significant correlation between duration of diabetes and quality of sleep, independent of other variables. It is important for physicians to address the quality and duration of sleep in patients with type 2 diabetes.
Diabetes is a global health problem with significant human, social, and economic impacts. More than 300 million people worldwide live with diabetes. Each year, approximately 7 million new patients develop diabetes. Sleep disturbances are common among individuals with diabetes. Patients with type 2 diabetes have been shown to have an increased prevalence of periodic breathing and time spent in rapid eye movement sleep. In addition, patients with diabetes are reported to have higher rates of insomnia, excessive daytime sleepiness and a higher incidence of restless leg syndrome. A majority of these patients may also have obstructive sleep apnea (OSA) [1]. OSA and diabetes mellitus share several risk factors, including advanced age and obesity [2]. Diabetes and OSA appear to be associated independent of the degree of adiposity [3-9]. Because both diabetes and OSA are associated with increased cardiovascular morbidity and mortality, it is possible that the presence of both conditions results in additive or even synergistic health risks [10]. To promote better management of type 2 diabetes, sleep disorders must be addressed in these patients. Identifying sleep disturbances in type 2 diabetics and treating them early may improve overall prognosis and quality of life.
The present study was carried out to identify the prevalence of sleep disturbances among patients with type 2 diabetes in a southeast Asian Indian population and to determine the predictors of sleep impairment in this population using a validated instrument, the Pittsburgh Sleep Quality Index (PSQI).
The study was carried out at the Sri Ramachandra Medical Center, a tertiary-care teaching hospital in Chennai, South India. The study population was comprised of type 2 diabetes patients attending the Endocrine Outpatient Clinic. The total study sample included 120 patients; 96 had type 2 diabetes alone, and 24 had type 2 diabetes with co-existing hypertension. Patients who were diagnosed as having type 2 diabetes within the past year were excluded from the study, as were patients with other comorbidities like malignancy, renal disorders, lung diseases, liver diseases, thyroid disorders, and cardiac diseases. Smokers and alcoholics were also excluded from the study. Protocols and informed consent documents were approved by the Institutional Ethics Board in accordance with International and National Guidelines. All participants signed an informed consent form before inclusion in the study. The study period extended from September 2010 to February 2011.
For each patient, we recorded data regarding age, gender, duration of diabetes, and use of medications. Height, weight, and waist circumference were measured in all participants; body mass index (BMI, kg/m2) was calculated as weight divided by height squared. A detailed physical examination was then performed. All vital signs were recorded. Fasting blood glucose (FBG), postprandial blood glucose (PPBG), and glycosylated hemoglobin (HbA1c) values were available for all the patients and were recorded from their case sheets. All the study patients were on anti-diabetic medications, either insulin or oral hypoglycemic agents or both.
In all patients, quality of sleep was evaluated by administering the PSQI through an interview. The PSQI is a self-report questionnaire that assesses sleep quality and quantity over a monthlong period [11]. The questionnaire consists of 19 self-rated questions and 5 questions that should be answered by bedmates or roommates. The latter questions were used only for clinical information and were not used in the scoring. The 19 questions were categorized into 7 components, which are graded on a scale that ranges from 0 to 3. The PSQI components are as follows: subjective sleep quality (C1), sleep latency (C2), sleep duration (C3), habitual sleep efficiency (C4), sleep disturbances (C5), use of sleeping medication (C6), and daytime dysfunction (C7). The sum of scores for these 7 components yields one global PSQI score, which ranges from 0 to 21, where the highest score indicates the worst sleep quality. A global PSQI ≥5 has a diagnostic sensitivity of 89.6 and specificity of 86.5 in distinguishing "poor sleepers" (PSQI ≥5) from "good sleepers" (PSQI <5) [12]. The design of the PSQI is such that the items and the component scores represent standard areas of clinical focus in patients with reported sleep problems [13].
Statistical analysis was conducted with the SPSS version 17.1 (SPSS Inc., Chicago, IL, USA). Quantitative variables were expressed as mean±standard deviation, and qualitative variables were expressed as percentage values. The scatter plot diagram was used to find the correlation of various variables with the global PSQI score. The R2 value was obtained from the scatter plot from which the P value was determined. Logistic regression analysis was then applied to explore relationships between PSQI and other variables like age, gender, BMI, duration of diabetes, HbA1c, and choice of medications. For comparison between the three groups, we used the Kruskal-Wallis test. All tests were two-tailed, and we set the level of significance at P values of less than 0.05.
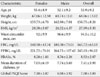
One hundred twenty patients completed the PSQI questionnaire and were included in the study. The demographic characteristics of these patients are listed in Table 1. Our sample consisted of 55 females and 65 males. The mean age of the participants was 53.9 years (standard deviation [SD], 9.2 years), and the average BMI was 27.09 kg/m2 (SD, 5.3 kg/m2). Fifty-one patients (43%) were overweight (BMI, 25 to 29.9 kg/m2), 28 (23%) were obese (BMI ≥30 kg/m2), and the remaining 41 (34%) had a BMI <25 kg/m2.
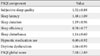
The mean PSQI score of the study patients was 7.08 (SD, 3.89), and 69% (n=83) had a PSQI score ≥5, indicating poor quality of sleep. Individual components of PSQI are shown in Table 2. Of the seven components of PSQI, sleep latency was the most affected in our study population. Average sleep latency was 35.3 minutes (SD, 33.84), and the mean duration of sleep was 375.04 minutes (SD, 68.40). Global PSQI score did not differ significantly in hypertensive patients (7.04±3.17) compared to normotensive patients (7.17±4.09).
A significant correlation was found between the duration of diabetes and the global PSQI score (R2=0.181, P<0.05), indicating that the duration of diabetes had a strong influence on sleep in our study sample (Fig. 1). This correlation was independent of other variables like age, gender, BMI, HbA1c, or choice of medications, as shown in Table 3. Duration of diabetes also had weaker correlations with the individual components of the PSQI, of which subjective sleep quality correlated the most (R2=0.123); however, this correlation was not statistically significant. No significant correlation existed between HbA1c and global PSQI score. In contrast, there was a positive trend (R2=0.03) between the two factors in patients with uncontrolled diabetes (HbA1c ≥7%). Other variables like BMI, age, gender, and choice of medications did not correlate with global PSQI score.
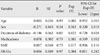
Further analysis was done based on medications. Participants were divided into three groups: 1) patients who took only oral hypoglycemic agents (OHAs); 2) patients who took only insulin; and 3) patients who took both. Their respective PSQI scores were 6.43±3.63 (only OHAs), 8.00±4.19 (only insulin), and 8.43±4.08 (both OHAs & insulin). Although the first group (only OHAs) had a low overall PSQI score compared to the other two groups, there was no statistically significant difference among the three groups in terms of individual components (like subjective sleep quality, sleep latency) or global PSQI score (Table 4).
Our results suggest that there is a high prevalence of sleep dysfunction in patients with type 2 diabetes. In our study, a high proportion (69%) of type 2 diabetics had a PSQI score ≥5, which suggests reduced and disturbed sleep in these patients. Among the individual components of PSQI, sleep latency was the most affected, followed by sleep quality and duration. Duration of diabetes was the greatest predictor of sleep disturbances among our study sample, while age, gender, BMI, insulin or OHA use or HbA1c did not correlate with sleep dysfunction. Mean duration of sleep per day was 6 hours and 15 minutes, suggesting that a majority of type 2 diabetics suffer from sleep disturbances.
Sleep disturbances are more common among diabetics than they are in the general population. Almost 50% of these patients have sleep apnea [14]; among obese patients with type 2 diabetes, the prevalence of sleep apnea has been estimated at 86% [15]. Sleep disturbances and sleep loss are also implicated in insulin resistance, a precursor to type 2 diabetes. Individuals who sleep more than 8 hours per day or less than 7 hours per day are at a modestly increased risk of all-cause mortality, cardiovascular disease, and developing symptomatic diabetes [16].
Sleep apnea and sleep-disordered breathing contribute to metabolic derangements such as glucose intolerance and insulin resistance independent of obesity [3-9]; accumulating evidence suggests that they may be independent risk factors for hypertension and cardiovascular disease [17-19]. However, the relationship between sleep-disordered breathing (SDB) and diabetes is not clearly understood. A few studies have attempted to evaluate the cross-sectional relationship, but they have had mixed results. In their study, Reichmuth et al. [20] concluded that SDB contributes to weight gain and obesity; these changes lead to increased insulin resistance, which in turn increases the patient's risk of developing diabetes. In a clinic-based study, Vgontzas and colleagues [8] found that patients with sleep-disordered breathing had significantly higher fasting glucose and insulin levels compared with a group of weight-matched control participants. They also suggested that the glucose intolerance and insulin resistance associated with sleep-disordered breathing may be intermediate in the putative causal pathway to increased cardiovascular morbidity and mortality [21,22].
Stoohs et al. [5] reported that worsening insulin sensitivity and SDB in a group of 50 "healthy, normotensive individuals" was completely accounted for by their increased BMI. Another study [3] found a relationship between fasting insulin level and increasing apnea-hypopnea index (AHI) in patients with BMI of 29 or greater but not in those with lower BMI. In our study, BMI did not have a significant correlation with global PSQI score. However, our results showed a trend toward a positive association of BMI ≥25 kg/m2 with global PSQI score (R2=0.02), indicating that BMI may affect sleep quality in overweight and obese type 2 diabetes patients. This finding is in accordance with the study by Vorona et al. [23], who found a similar relationship. Our analysis also suggested that the choice of medications does not have a significant influence on sleep quality in type 2 diabetes patients.
Interestingly in our study, duration of diabetes significantly correlated with global PSQI score. Patients with a longer duration of diabetes had poor sleep quality independent of other variables. The reason for this is unclear but could be due to multiple factors, including neuropathy and nocturia, which are more prevalent in long-term diabetics. We did not find a significant correlation between HbA1c and global PSQI score. However, in patients with uncontrolled diabetes (HbA1c ≥7%), a mild positive association existed between the two, indicating poor quality of sleep in these patients. The study by Nakajima et al. [24] supported our observations by reporting a short sleep duration in individuals with high HbA1c levels. Sleep deprivation in these patients may in turn worsen their diabetic state by 1) triggering the secretion of stress hormones and proinflammatory cytokines, leading to glucose intolerance and decreased insulin sensitivity [8]; 2) increasing sympathetic nervous system activity [25,26], which may impair glucose regulation via the lipolytic effects of adrenergic stimulation of visceral adipose tissue [27]; and 3) decreasing levels of the satiety hormone leptin and increasing the hunger hormone ghrelin, thereby increasing hunger and food intake [28]. Recent evidence also suggests that sleep durations of 6 hours or less or 9 hours or more are associated with increased prevalence of diabetes and impaired glucose tolerance (IGT) [29]. The experimental restriction of sleep to 4 hours per night for 6 nights produced IGT in healthy young adults [30].
A number of studies have shown that treatment of sleep disorders may help in attaining better glycemic control in type 2 diabetics. Continuous positive airway pressure (CPAP) therapy significantly improved insulin sensitivity in 40 patients with sleep apnea. This improvement was observed within 2 days of treatment and was sustained over 3 months [31]. Insulin responsiveness increased by 28% in patients with type 2 diabetes after 4 months of CPAP therapy [32]. In patients with type 2 diabetes, postprandial glucose levels were significantly reduced after approximately 3 months of CPAP therapy that had been initiated to treat sleep apnea. HbA1c levels were significantly reduced in patients with HbA1c >7% [33]. Continuous blood glucose monitoring of type 2 diabetes patients on CPAP therapy for an average of 41 days demonstrated lower and more stable nocturnal glucose levels [34].
Based on the current evidence, a strong association exists between sleep and glucose regulation. Our study identified a higher prevalence of sleep dysfunction in type 2 diabetics, and there was a strong association between duration of diabetes and the quality of sleep in these individuals. Sleep latency was the most affected symptom among these patients. Our study reiterated the importance of sleep evaluation in type 2 diabetes patients, as better sleep quality in these patients may improve the overall prognosis of the disease by significantly reducing cardiovascular risks. Furthermore, treatment of sleep apnea improves insulin sensitivity and could benefit the metabolic profiles of these patients.
Our study has several limitations. First, we did not have a healthy non-diabetic control group in our study to facilitate a direct comparison. Second, we did not have polysomnographic measures of sleep in this study, so it was not possible to detect sleep disorders like obstructive sleep apnea. Third, we could not exclude the possibility of confounding effects by unmeasured variables, such as a sedentary lifestyle or poor diet. Finally, we did not find a significant correlation of HbA1c or BMI with sleep quality, which can be attributed to the smaller sample size of our study.
In summary, our study suggested that southeast Asian Indian patients with type 2 diabetes had a higher prevalence of sleep dysfunction relative to the general population. Our analysis also indicated that the duration of diabetes was the greatest predictor of sleep disturbances in diabetic patients. Identifying sleep disturbances in this population and treating them early could reduce significant cardiovascular risk.
Figures and Tables
ACKNOWLDEGMENTS
This research was not supported by any specific grants from any funding agencies in the public, commercial or not-for-profit sector.
The authors would like to thank Ms. R. Kavitha, Mrs. Vijaya Rani, Mr. D. R. Nagendra Kumar, Mr. Gowri Shankar, and Ms. Hepsi Kirubha for their contributions to this study. We also thank all study participants and health workers who participated in the study for their excellent cooperation.
References
1. Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008. 133:496–506.
2. Early release of selected estimates based on data from the January-June 2003 National Health Interview Survey. Centers for Disease Control and Prevention. cited 2005 Jan 20. Available from: http://www.cdc.gov/nchs/about/major/nhis/released200312.htm.
3. Strohl KP, Novak RD, Singer W, Cahan C, Boehm KD, Denko CW, Hoffstem VS. Insulin levels, blood pressure and sleep apnea. Sleep. 1994. 17:614–618.
4. Grunstein RR, Stenlof K, Hedner J, Sjostrom L. Impact of obstructive sleep apnea and sleepiness on metabolic and cardiovascular risk factors in the Swedish Obese Subjects (SOS) Study. Int J Obes Relat Metab Disord. 1995. 19:410–418.
5. Stoohs RA, Facchini F, Guilleminault C. Insulin resistance and sleep-disordered breathing in healthy humans. Am J Respir Crit Care Med. 1996. 154:170–174.
6. Strohl KP. Diabetes and sleep apnea. Sleep. 1996. 19:S225–S228.
7. Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000. 118:580–586.
8. Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales A, Chrousos GP. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000. 85:1151–1158.
9. Elmasry A, Lindberg E, Berne C, Janson C, Gislason T, Awad Tageldin M, Boman G. Sleep-disordered breathing and glucose metabolism in hypertensive men: a population-based study. J Intern Med. 2001. 249:153–161.
10. Boyer S, Kapur V. Obstructive sleep apnea: its relevance in the care of diabetic patients. Clin Diabetes. 2002. 20:126–132.
11. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989. 28:193–213.
12. Fiorentini A, Valente R, Perciaccante A, Tubani L. Sleep's quality disorders in patients with hypertension and type 2 diabetes mellitus. Int J Cardiol. 2007. 114:E50–E52.
13. Aloba OO, Adewuya AO, Ola BA, Mapayi BM. Validity of the Pittsburgh Sleep Quality Index (PSQI) among Nigerian university students. Sleep Med. 2007. 8:266–270.
14. Einhorn D, Stewart DA, Erman MK, Gordon N, Philis-Tsimikas A, Casal E. Prevalence of sleep apnea in a population of adults with type 2 diabetes mellitus. Endocr Pract. 2007. 13:355–362.
15. Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, Wadden TA, Kelley D, Wing RR, Sunyer FX, Darcey V, Kuna ST. Sleep AHEAD Research Group. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009. 32:1017–1019.
16. Phillips B, Hening W, Britz P, Mannino D. Prevalence and correlates of restless legs syndrome: results from the 2005 National Sleep Foundation Poll. Chest. 2006. 129:76–80.
17. Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000. 283:1829–1836.
18. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000. 342:1378–1384.
19. Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001. 163:19–25.
20. Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005. 172:1590–1595.
21. Hammond EC. Some preliminary findings on physical complaints from a prospective study of 1,064,004 men and women. Am J Public Health Nations Health. 1964. 54:11–23.
22. Wingard DL, Berkman LF, Brand RJ. A multivariate analysis of health-related practices: a nine-year mortality follow-up of the Alameda County Study. Am J Epidemiol. 1982. 116:765–775.
23. Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005. 165:25–30.
24. Nakajima H, Kaneita Y, Yokoyama E, Harano S, Tamaki T, Ibuka E, Kaneko A, Takahashi I, Umeda T, Nakaji S, Ohida T. Association between sleep duration and hemoglobin A1c level. Sleep Med. 2008. 9:745–752.
25. Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000. 279:H234–H237.
26. Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab. 1999. 84:1979–1985.
27. Lonnqvist F, Thorne A, Large V, Arner P. Sex differences in visceral fat lipolysis and metabolic complications of obesity. Arterioscler Thromb Vasc Biol. 1997. 17:1472–1480.
28. Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004. 141:846–850.
29. Wilcox I, McNamara SG, Collins FL, Grunstein RR, Sullivan CE. "Syndrome Z": the interaction of sleep apnoea, vascular risk factors and heart disease. Thorax. 1998. 53:Suppl 3. S25–S28.
30. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999. 354:1435–1439.
31. Harsch IA, Schahin SP, Radespiel-Troger M, Weintz O, Jahreiss H, Fuchs FS, Wiest GH, Hahn EG, Lohmann T, Konturek PC, Ficker JH. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004. 169:156–162.
32. Brooks B, Cistulli PA, Borkman M, Ross G, McGhee S, Grunstein RR, Sullivan CE, Yue DK. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994. 79:1681–1685.
33. Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005. 165:447–452.
34. Dawson A, Abel SL, Loving RT, Dailey G, Shadan FF, Cronin JW, Kripke DF, Kline LE. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J Clin Sleep Med. 2008. 4:538–542.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download