Abstract
Background
There is growing concern regarding the increased incidence of bladder cancer in diabetic patients using pioglitazone. This study aimed to investigate the association between bladder cancer and the use of pioglitazone in Korean diabetics.
Methods
This retrospective, matched case-control study included a case group (n=329) of diabetic patients with bladder cancer who presented at the Severance Hospital from November 2005 to June 2011. The control group consisted of patients without bladder cancer (1:2 ratio matching for sex and age, n=658) who were listed on the Severance Hospital diabetes registry.
Results
The percentage of subjects who had ever used pioglitazone was significantly lower in the case group than in the control group (6.4% vs. 15.0%, P<0.001). Multivariate conditional logistic analysis revealed that independent factors affecting bladder cancer were smoking (odds ratio [OR], 11.64; 95% confidence interval [CI], 6.56 to 20.66; P<0.001), coexisting cancer (OR, 6.11; 95% CI, 2.25 to 16.63; P<0.001), and hemoglobin levels (OR, 0.78; 95% CI, 0.69 to 0.88; P<0.001). The OR of the history of pioglitazone use was 2.09 and was not significantly different between the two groups (95% CI, 0.26 to 16.81; P=0.488).
Conclusion
A relationship between pioglitazone use and incidence of bladder cancer was not observed in Korean diabetic patients. This suggests that the risk for bladder cancer in Korean diabetic subjects treated with pioglitazone might be different from that of Caucasian populations. Large-scale, well-designed and multi-center studies are needed to further evaluate this relationship.
The expression of peroxisome proliferator activator-receptors (PPAR)-γ in normal uroepithelilal cells or in bladder cancer cells may cause antiproliferative effects [1] or potential tumorigenicity [2]. In preclinical animal studies, transitional papilloma and carcinoma originated from the urothelium in the urinary bladder and renal pelvis of Sprague-Dawley Wistar and Fisher strain rats of both sexes treated with 5/6 PPAR dual agonists (α/γ) and one γ agonist (pioglitazone). These findings prompted a similar epidemiologic study in humans [3], and recent human clinical trials have caused growing concern regarding pioglitazone use in subjects with type 2 diabetes [4-6]. An interim report from a longitudinal cohort study concluded that short-term use of pioglitazone was not significantly associated with an increased incidence of bladder cancer, but that using pioglitazone longer than 2 years was weakly associated with increased risk of bladder cancer [6]. Another epidemiological study conducted in France suggested that use of pioglitazone is associated with risk of bladder cancer [5], resulting in a ban of pioglitazone in France. Based on these findings, the Food and Drug Administration of the United States of America has been closely tracking data from an ongoing, 10-year epidemiological study.
There are numerous ethnic and cultural differences between Korean populations and Caucasian populations, including differences in medical milieu. Therefore it is essential to evaluate the association between pioglitazone use and bladder cancer in Koreans. This study investigated the association between the pioglitazone use and incidence of bladder cancer in diabetic Koreans.
In this retrospective, case-control study, clinical and laboratory data from Severance Hospital at the Yonsei University College of Medicine were retrieved from electronic medical records. The study protocol was approved by the Ethics Committee of the Yonsei University College of Medicine. Patients were eligible for enrollment if the following criteria were met: 1) older than 20 years of age; 2) presenting with type 2 diabetes from November 2005 to June 2011; 3) bladder cancer diagnosis confirmed by cytology. Among the diabetic patients with bladder cancer, those who were dependent on pioglitazone at the time of or before the detection of bladder cancer were included. Patients who were 1) diagnosed with bladder cancer prior to the diagnosis of diabetes, 2) treated with pioglitazone after being diagnosed with bladder cancer, or 3) found to have benign pathology results were excluded. This study took into account different classes of anti-diabetic agents, potential confounding variables believed to be associated with the risk of bladder cancer (e.g., age, sex, duration of diabetes, obesity, alcohol, smoking, and anti-diabetic agents), and potential underlining medical conditions (e.g., malignancy, renal insufficiency with HbA1c, hemoglobin, albumin, total cholesterol). Records and data were reviewed for validation by two endocrinologists.
The population of subjects who were free of bladder cancer was supplemented with a case-control study from the Severance diabetes registry to avoid incomplete or missing data on potential confounders. For each diabetic subject with bladder cancer and a history of pioglitazone use, one control was randomly selected by SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) at the medical research supporting section of Yonsei University, after 1:2 ratio-matching for sex and age. Diabetic subjects on pioglitazone were classified into either the case group with bladder cancer or the control group without bladder cancer. Members of the case group and control group were further categorized into the following subgroups based on the type of anti-diabetic drug they were using: pioglitazone, other thiazolidinedione, metformin, sulfonylurea, insulin, α-glucosidase inhibitors, dipeptidyl peptidase (DPP)-4 inhibitors, and no medication.
Anthropometric and biochemical variables were measured. HbA1c was measured by high performance liquid chromatography using Variant II Turbo (Bio-Rad Laboratories, Hercules, CA, USA). An abbreviated modification of diet in renal disease study equation was used to estimate glomerular filtration rate (GFR) as follows: estimated GFR (mL/min/1.73 m2)=186×(creatinine/88.4)-1.154×(age)-0.203×(0.742, if female).
Confounding variables were determined based on habits at the time of first diagnosis of bladder cancer. Smoking habits were categorized into never smoked, current smoker, and ex-smoker if they quit more than 1 year prior to the study. Smoking frequency was calculated as packs per year (PPY), where 1 PPY=20 cigarettes/day for 1 year. Alcohol consumption was defined as alcoholic intake greater than 20 g/day, as stated in the World Health Organization guidelines. The cumulative dose of pioglitazone was calculated by multiplying daily doses of pioglitazone (15 mg) by the number of days prescribed. The cumulative doses of pioglitazone in the case group was calculated based on the number of doses prescribed according to Severance Hospital medical records from the first day of the pioglitazone prescription to the day of bladder cancer diagnosis.
Statistical parameters were calculated using PASW Statistics version 18 (SPSS, Chicago, IL, USA) and P<0.05 was considered significant. Continuous variables with a normal distribution were expressed as mean±standard deviation and compared using an independent t-test. Pearson's chi-square test and Fisher's exact tests were employed for comparison of categorical variables. A multivariate conditional logistic regression model was used to determine the factors associated with bladder cancer in diabetes patients, and the skewed data were summarized using median values and an inter-quartile range.
Of the 53,569 subjects presenting with type 2 diabetes in Severance Hospital from November 2005 to June 2011, 507 subjects were diagnosed with both bladder cancer and diabetes. Of these 507 subjects, 178 were excluded, leaving 329 patients who fulfilled the aforementioned criteria for final inclusion in the study. Of the 53,569 eligible subjects regardless of bladder cancer, 6,069 subjects had never used pioglitazone. A total of 658 age- and sex-matched diabetics without bladder cancer were enrolled as the control group.
Type 2 diabetic subjects with and without bladder cancer were classified into the case group and the control group, respectively (Fig. 1). Baseline characteristics of the participants are shown in Table 1. Age (69.4±9.9 years) and male dominant gender ratio (5.32:1) were similar between the two groups after matching for sex and age. The duration of diabetes (9.66±16.0 years vs. 8.66±8.7 years) and body mass index (24.0±3.1 kg/m2 vs. 25.1±12.0 kg/m2) were not significantly different between the two groups. History of alcohol use (51.9% vs. 27.6%, P<0.001), smoking (64.1% vs. 11.7%, P<0.001), and proportions of other coexisting cancers (11.0% vs. 2.0%, P<0.001) were significantly higher in the case group than in the control group. However, smoking levels as assessed by PPY were not different between the two groups (26.8±34.1 vs. 26.4±17.2, P=0.899). The percentage of subjects who had ever used pioglitazone (6.4% vs. 15.0%, P<0.001), the number of pioglitazone prescription days (555.4±606.6 days vs. 770.6±772.8 days, P=0.216; median 363.5 [interquartile range, 100.3 to 632.3] vs. median 485.0 [interquartile range, 230.0 to 1,135.0], P=0.254), and the cumulative dose of pioglitazone (5,876.9±5,500.3 mg vs. 11,558.6±10,842.6 mg, P=0.001; median 5,400.0 [interquartile range, 1,350.0 to 8,861.3] vs. 7,275.0 [interquartile range, 3,450.0 to 17,025.0], P=0.033) were significantly lower in the case group than in the control group. Hemoglobin (12.7±5.1 g/dL vs. 13.4±2.5 g/dL, P=0.008) and albumin (3.97±0.7 mg/dL vs. 4.14±0.6 mg/dL, P<0.001) were significantly lower in the case group than in the control group, but HbA1c (7.22±1.2% vs. 7.41±4.9%), total cholesterol, aspartate aminotransferase or alanine aminotransferase levels were not (Table 1).
An analysis of anti-diabetic medications used in the case group revealed that sulfonylurea (46.4%) is the most widely used, followed by no medication (17.4%), insulin (12.6%), metformin only (8.5%), rosiglitazone (5.7%), and pioglitazone (6.4%). In the control group, most subjects did not take medication (26.6%), followed by sulfonylurea (20.5%), metformin only (14.0%), insulin (11.8%), and pioglitazone (15.0%). The proportion of patients undergoing combination therapy of metformin and pioglitazone was not significantly different between the two groups. The proportion of pioglitazone use was significantly different between the case group and the control group (6.4% vs. 15.0%, P<0.001) (Fig. 2).
A multivariate conditional logistic regression analysis revealed that independent factors affecting bladder cancer were smoking (odds ratio [OR], 11.6; 95% confidence interval [CI], 6.56 to 20.66; P<0.001), coexisting cancer (OR, 6.11; 95% CI, 2.25 to 16.63; P<0.001), and low level of hemoglobin (OR, 0.78; 95% CI, 0.69 to 0.88; P<0.001) (Table 2). The OR of pioglitazone use was only 2.09 and was not significantly different between the two groups (95% CI, 0.26 to 16.81; P=0.488). Moreover, the OR of cumulative doses of pioglitazone was 1.00 (95% CI, 1.00 to 1.00; P=0.041). Cumulative doses of pioglitazone were unrelated to bladder cancer in this study (Table 2).
A growing number of epidemiologic evidence suggests that there is a significantly higher risk of several malignancies, such as pancreatic, esophageal, breast, and colon cancer in subjects with diabetes [7,8]. Clinical trials and several retrospective registry analyses indicate that diabetic subjects who have used pioglitazone may have an increased risk of bladder cancer [4,6,9-12]. Although pioglitazone insignificantly increased bladder cancer risk in the Taiwanese population [12], characteristics associated with bladder cancer in Taiwanese subjects are different from those in Caucasian subjects. In Caucasian populations there was a weakly increased risk of bladder cancer after more than 2 years of pioglitazone therapy [6], whereas bladder cancer in Taiwanese diabetics patients occurred within 2 years of the initiation of therapy and no patients with a cumulative dose >28,000 mg developed bladder cancer [12]. Based on these findings, Tseng [12] suggested that pioglitazone has an immediate effect on the risk of bladder cancer or late pioglitazone use in patients with a high risk of bladder cancer.
According to the Korean Central Cancer Registry (KCCR) [13], the age-standardized rate (ASR) of bladder cancer in Korea decreased between 2004 and 2008; the ASRs of bladder cancer across both genders for 2004, 2005, 2006, 2007, and 2008 were 5.2, 5.2, 5.0, 5.0, and 4.9/100,000, respectively. In the male population, the ASRs for 2004 through 2008 were 10.0, 10.0, 9.8, 9.5, and 9.2/100,000, respectively (Supplementary Fig. 1). Pioglitazone was first launched in 2003 in Korea. Its market share in the Korean oral hypoglycemic agent market started relatively low (ranging from 3% to 5% in 2007) and increased slightly to 7% in 2008, when the patent on original pioglitazone expired and the generic drug launched. The relationship between market share, increased pioglitazone prescriptions, and the ASR of bladder cancer in Korea seems to indicate there is not a direct correlation between bladder cancer and pioglitazone. This simple proof-of-concept data analysis suggests that pioglitazone may not be associated with the occurrence of bladder cancer, and the present study tested this hypothesis.
Considering the differences in ethnicity and medical milieu between the Korean population and the Caucasian population, and using simple proof-of-concept data analysis [14,15], the authors hypothesized that pioglitazone use might not be associated with bladder cancer. The present study primarily focused on the characteristics of Korean type 2 diabetic patients with bladder cancer and on the statistical association between pioglitazone use and the occurrence of bladder cancer using a case-control study. This study used data from the Severance Hospital diabetes registry to avoiding incomplete or missing information about potential confounders. The study has three main findings. First, diabetic patients with bladder cancer in the Severance Hospital data pool were relatively old (69.4±9.9 years) and predominantly male (5.32 ratio over female), which is similar to data from the Korea Central Cancer Registry. Second, contrary to what was expected, the proportion of patients using pioglitazone, as well as the total cumulative dose of pioglitazone, was significantly higher in diabetics without bladder cancer. In a multivariate logistic analysis, the OR of pioglitazone use was 2.09 and was not significantly different between the two groups (95% CI, 0.26 to 16.81, P=0.488). Third, in accordance with previous reports, independent variables associated with bladder cancer included coexisting malignancy, smoking, and anemia. It is well-known that smoking is the primary risk factor of bladder cancer, especially in males [16]. Recent reports on the risk of bladder cancer among diabetic patients treated with pioglitazone in Caucasian and Asian populations [6,12] adopted Cox proportional hazards models for all calculations of the hazard ratio of bladder cancer and did not consider smoking history or current smoking status. This study performed a multiple conditional regression analysis adjusting for smoking, a well-known risk factor of bladder cancer. In this study, pioglitazone use was not significantly different between the bladder cancer and cancer-free group; however, the OR of smoking was 11.6, confirming that smoking is an important risk factor of bladder cancer. Confirmation of smoking status is essential in accurately assessing the incidence of bladder cancer. In addition, the fact that diabetic patients with bladder cancer were significantly more likely to have other cancers could be attributed to smoking. Establishing a cause-effect relationship regarding anemia was unsuccessful due to the limitations of a cross-sectional study, but it is highly probable that the anemia was an epiphenomenon, possibly due to bladder bleeding in cancer patients.
Unlike results from previous studies, this study found the incidence of bladder cancer among diabetic patients to be unrelated to pioglitazone use. Possible explanations for this include differences in pioglitazone dosing, in addition to an absolutely low incidence of bladder cancer. The typical dose of pioglitazone in South Korea is 15 mg, while the typical dose in Western nations is of 30 to 40 mg. Indeed, in the case group and control group, all subjects had taken 15 mg of pioglitazone daily, with the exception of a single subject who had taken 7.5 mg of pioglitazone daily. In addition, the age-adjusted bladder cancer incidence in the U.S. was 37.7/100,000 in 2007 [17], and the age-standardized rate in South Korea was 9.2/100,000 in 2008 [13]. Only 165 patients with bladder cancer were included in the previously mentioned Taiwanese study, among 54,928 patients who were analyzed. This study, however, included 329 cases of bladder cancer even though the data came from only one study center.
This case-control study had several limitations. First, there were incomplete or missing data for several variables known to be associated with bladder cancer, such as history of exposure to chemical toxins and presence of comorbid conditions such as urinary disease. Also, the cumulative dose of pioglitazone was calculated as the amount of drug prescribed since the first prescription made at the tertiary hospital until the day of diagnosis of bladder cancer. These calculations were based on a review of medical records, which caused difficulties in calculating pioglitazone intake prior to visiting the tertiary center. As a result, the actual cumulative dose of pioglitazone may have been underestimated. In addition, a survival analysis comparing the incidence of bladder cancer between diabetic patients with and without the history of pioglitazone use was not included in this study. Therefore, this cross-sectional study might be confounded by the duration of observation for each group. Lastly, because this study was a retrospective analysis, there may be a recall bias.
Data from 3,500 bladder cancer patients across several university hospitals, which manage over 35% of all bladder cancer patients in South Korea, are currently being analyzed in an attempt to overcome the limitations of the current study. In addition, a request has been made to the National Health Insurance Database to provide data for all pioglitazone prescriptions written to diabetic patients with or without bladder cancer, after which a hazard ratio will be calculated using the Cox regression model. This ongoing study aims to investigate the time to diagnosis of bladder cancer, cumulative dose of pioglitazone, presence of other cancers, and smoking and alcohol history in the patient registry by enrolling approximately 200,000 patients who have been diagnosed with diabetes and are receiving care across several representative hospitals. Thus, in the future this ongoing study will conduct a more accurate assessment of the association between the pioglitazone use and incidence of bladder cancer among diabetic patients.
In summary, this study did not observe a significantly increased risk of bladder cancer among patients treated with pioglitazone in Korean diabetics. However, the study also is unable to absolutely exclude the possibility of a relationship between pioglitazone and bladder cancer because the OR for pioglitazone use was relatively high (2.09, P=0.484). This suggests that the risk of bladder cancer in Korean diabetics treated with pioglitazone might be different from that of Caucasian population.
Figures and Tables
Supplementary Fig. 1
Frequency of bladder cancer and pioglitazone market in Korea. (A) Both. (B) Male. (C) Female. The crude rate (CR), a rate based on the frequency of cancer in the entire population, is calculated as follow: CR (per 100,000 person-years)=(Number of events/Corresponding person-years of observation)×100,000. The an age-standardized rate (ASR) is a weighted average of crude age-specific rates, where the CRs are calculated for different age groups and the weights are the proportions of persons in the corresponding age groups of a standard population.

Table 1
Demographics and confounder variables
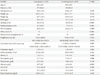
Continuous variables are presented as mean±standard deviation or median (interquartile range). Also categorical variables are presented as number and percent (%).
DM, diabetes mellitus; BMI, body mass index; PPY, pack per years; IQR, interquartile range; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
References
1. Yoshimura R, Matsuyama M, Segawa Y, Hase T, Mitsuhashi M, Tsuchida K, Wada S, Kawahito Y, Sano H, Nakatani T. Expression of peroxisome proliferator-activated receptors (PPARs) in human urinary bladder carcinoma and growth inhibition by its agonists. Int J Cancer. 2003. 104:597–602.
2. Cohen SM. Effects of PPARgamma and combined agonists on the urinary tract of rats and other species. Toxicol Sci. 2005. 87:322–327.
3. Aoki T. Current status of carcinogenicity assessment of peroxisome proliferator-activated receptor agonists by the US FDA and a mode-of-action approach to the carcinogenic potential. J Toxicol Pathol. 2007. 20:197–202.
4. Piccinni C, Motola D, Marchesini G, Poluzzi E. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011. 34:1369–1371.
5. Stephenson J. Diabetes drug may be associated with increase in risk of bladder cancer. JAMA. 2011. 306:143.
6. Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP Jr, Vaughn DJ, Nessel L, Selby J, Strom BL. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011. 34:916–922.
7. Suh S, Kim KW. Diabetes and cancer: is diabetes causally related to cancer? Diabetes Metab J. 2011. 35:193–198.
8. Ogunleye AA, Ogston SA, Morris AD, Evans JM. A cohort study of the risk of cancer associated with type 2 diabetes. Br J Cancer. 2009. 101:1199–1201.
9. Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. PROactive investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005. 366:1279–1289.
10. Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR. PROactive investigators. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf. 2009. 32:187–202.
11. MacKenzie T, Zens MS, Ferrara A, Schned A, Karagas MR. Diabetes and risk of bladder cancer: evidence from a case-control study in New England. Cancer. 2011. 117:1552–1556.
12. Tseng CH. Pioglitazone and bladder cancer: a population-based study of Taiwanese. Diabetes Care. 2012. 35:278–280.
13. National Cancer Center. Annual report of cancer statistics in Korea in 2008. Available from: http://ncc.re.kr/manage/manage03_033_view.jsp?bbsnum=209&hSelSearch=&hTxtKeyword=¤t_page=1&cd=null (updated 2010 Dec 28).
14. Ko SH, Kim SR, Kim DJ, Oh SJ, Lee HJ, Shim KH, Woo MH, Kim JY, Kim NH, Kim JT, Kim CH, Kim HJ, Jeong IK, Hong EK, Cho JH, Mok JO, Yoon KH. Committee of Clinical Practice Guidelines, Korean Diabetes Association. 2011 Clinical practice guidelines for type 2 diabetes in Korea. Diabetes Metab J. 2011. 35:431–436.
15. Rhee SY, Woo JT. The prediabetic period: review of clinical aspects. Diabetes Metab J. 2011. 35:107–116.
16. Brennan P, Bogillot O, Cordier S, Greiser E, Schill W, Vineis P, Lopez-Abente G, Tzonou A, Chang-Claude J, Bolm-Audorff U, Jockel KH, Donato F, Serra C, Wahrendorf J, Hours M, T'Mannetje A, Kogevinas M, Boffetta P. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer. 2000. 86:289–294.
17. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011. 61:212–236.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download