Abstract
Postrenal transplantation diabetes mellitus (PTDM), or new-onset diabetes after organ transplantation, is an important chronic transplant-associated complication. Similar to type 2 diabetes, decreased insulin secretion and increased insulin resistance are important to the pathophysiologic mechanism behind the development of PTDM. However, β-cell dysfunction rather than insulin resistance seems to be a greater contributing factor in the development of PTDM. Increased age, family history of diabetes, ethnicity, genetic variation, obesity, and hepatitis C are partially accountable for an increased underlying risk of PTDM in renal allograft recipients. In addition, the use of and kinds of immunosuppressive agents are key transplant-associated risk factors. Recently, a number of genetic variants or polymorphisms susceptible to immunosuppressants have been reported to be associated with calcineurin inhibition-induced β-cell dysfunction. The identification of high risk factors of PTDM would help prevent PTDM and improve long-term patient outcomes by allowing for personalized immunosuppressant regimens and by managing cardiovascular risk factors.
The Sulwon Award for Scientific Achievement is the Korean Diabetes Association's highest scientific award and honors an individual who has excellently contributed to the progress in the field of diabetes and metabolism. Sulwon Award is named after an emeritus professor Eung Jin Kim, who founded Korean Diabetes Association.
Prof. Hyun Chul Lee received the third Sulwon Award at 2011 International Conference on Diabetes and Metabolism, November 10-12, 2011 at Seoul, Korea.
During the immediate period after solid organ transplantation, rejection of the transplanted organ as well as the short-term survival rate of the patient is of primary concern. Overcoming the shortcomings of currently available surgical techniques and immunosuppressants would allow for a longer life expectancy in more patients. Accordingly, greater emphasis has been placed on caring for chronic transplant-associated morbidities, which are the primary determinant of expectancy and quality of life in the long-term post-transplantation periods. The increased prevalence of macrovascular events in transplant recipients compared to non-transplant subjects remains a pivotal challenge to overcome. Among the established risk factors for the development of cardiovascular events in transplant recipients, attention and research has focused on abnormal glucose homeostasis in kidney transplant recipients. Therefore this review focuses on what is currently known about how genetics and pancreatic β-cell dysfunction related to the development of diabetes in kidney recipients.
Hyperglycemia after renal transplantation leads to the development of microvascular morbidity as well as accelerated macrovascular events, resulting in reduced recipient survival [1]. In this regard, aggravation of pre-existing diabetes as well as new-onset transplant-associated hyperglycemia encompassing new onset diabetes mellitus (DM), impaired fasting glucose (IFG), and impaired glucose tolerance (IGT) have been introduced as gluco-metabolic targets in an effort to reduce the risk of developing chronic transplant-associated morbidity and mortality by implementing proper management approaches during pre- and post-transplant stages [2]. Postrenal transplantation diabetes mellitus (PTDM), first described by Starzl et al. [3] in 1964, is defined as new-onset diabetes after solid organ transplantation. The definition of diabetes according to the American Diabetes Association (ADA) is a fasting plasma glucose [FPG] ≥7.0 mmol/L or a 2-hour post-load glucose ≥11.1 mmol/L, and this is routinely adopted when diagnosing PTDM. However, the incidence and prevalence of PTDM varies among studies depending on the criteria used to diagnose diabetes, the usage or kinds of immunosuppressants, characteristics of the subject population, and time after transplantation [4]. In the 1970s and early 1980s, steroids were considered the gold standard drug-of-choice and 40% to 60% of transplant recipients developed DM [5,6]. Even with the advent of cyclosporine A (CsA) in 1978, the prevalence of PTDM ranges widely from 5% to 45% [7-12], which is 2 to 9 times more than that of DM in an age- and sex-matched general population. A meta-analysis involving 19 observational studies and controlled trials reported that the rates of PTDM in the first post-transplantation year range from 2% to 50%. Two of the largest epidemiologic studies investigating the incidence of PTDM in subjects on the waiting list for renal transplantation and transplant recipients [13,14] demonstrated a 6% annual baseline incidence of new onset diabetes for the wait-listed subjects, and a 14% to 16% increase in the incidence of PTDM during the first post-transplantation year for transplant recipients. In the Korean population, it was previously reported that the overall incidence of PTDM was 39% at 1 year after transplantation and 35.1% at 7 years after transplantation [15]. Based on these results, PTDM might be especially accelerated in the first few post-transplantation months by the superimposition of transplant-related factors on baseline risks.
The risk factors associated with PTDM have been examined in many studies. Underlying baseline risk factors such as increased age, family history of diabetes, ethnicity, genetic variation, obesity, and hepatitis C are partially accountable for an increased underlying risk of PTDM in renal allograft recipients [16-18]. Non-modifiable risk factors of PTDM appear to be greater for patients who are older at the time of transplantation and have a familial history of diabetes [19]. Kidney transplant recipients older than 40 years of age are at greater risk of PTDM than younger recipients [11]. Gaining weight after transplantation, particularly among kidney transplant recipients, and infection with the hepatitis C virus [20] are modifiable risk factors for the development of PTDM. Similar to type 2 diabetes (T2D), weight gain after transplantation leads to insulin resistance [21] resulting in the development of PTDM.
In terms of transplant-related risk factors, usage and types of immunosuppressive agents are key to the aggravation or development of DM. PTDM is a major adverse effect of immunosuppressants. The diabetogenic effect of glucocorticoids, which has been well known since the early transplantation era, mainly results from both decreased peripheral glucose utilization and increased hepatic glucose production. The introduction of the 2nd generation of immunosuppressants of calcineurin inhibitors such as CsA and tacrolimus (Tac) into the field of transplantation has improved the outcome of organ transplants. However, their widespread therapeutic use is hindered by numerous reciprocal side effects shared between both drugs. Among them, new onset DM after transplantation, also known as PTDM, is of particular concern because of its frequent occurrence [22] and its associations with increased risk of cardiovascular diseases and lower survival rates.
Although the clinical features of T2D, such as insidious development, asymptomatic periods, etc., are similar to those of PTDM, some questions still remain regarding the incidence of PTDM among different ethnic groups. Interestingly, non-Caucasian kidney transplant recipients such as African-Americans, Hispanics, and Koreans are at greater risk of developing PTDM. These differences might be partially related to the pathophysiological development or aggravation of diabetes. The pathophysiologic nature of T2D is characterized by a progressive decline in pancreatic β-cell function and worsening insulin resistance, resulting in the failure of islets to respond to oral anti-diabetic drugs. However, the major determinant factors of PTDM remain controversial and there is some debate regarding whether the pathophysiology of PTDM is the result of decreased insulin secretion, increased insulin resistance, or both. For example, Ekstrand et al. [23] stated that insulin resistance and decreased insulin secretion may both be responsible, while Midtvedt et al. [24] suggested that insulin resistance may be the major contributor, and Shimizu et al. [25] reported that insulin resistance was improved after transplantation. Finally, in a well-designed 6-year prospective study, Hagen et al. [26] reported that impaired insulin secretion was the dominant mechanism for the development of PTDM. We have demonstrated that β-cell dysfunction was a major contributing factor to the development of PTDM in Korean subjects [15], which is consistent with Hagen et al.'s results. As we previously described, hyperglycemia after transplantation might be accelerated by the superimposition of transplant-related factors on baseline risks. Considering the characteristics of Korean patients with T2D, whose secretory β-cell dysfunction was a major contributing factor to the development and aggravation of hyperglycemia [27,28], different pharmacokinetic responses may augment the diabetogenic impact of immunosuppressants and superimpose upon conventional susceptible risks in the Korean population. In this regard, we also reported that the interaction between traditional genetic risk factors and transplant-associated risk factors such as calcineurin inhibitors plays an important role in the augmentation of established and new onset diabetes [29].
The association between PTDM and calcineurin inhibitor drugs is well-established. Previous in vitro studies on purified islets and insulin-producing β-cells have proposed several diabetogenic actions for CsA and Tac. Both drugs impair insulin secretion [30-33], decrease insulin content of the β-cell [34,35] and impair insulin transcription [36,37], although the primary mechanisms are not yet fully understood. In insulin-secreting cells, calcineurin is involved in the stimulation of insulin gene transcription through the activation of the transcription factor nuclear factor of activated T-cells (NFAT) [38]. Indeed, mice deficient in calcineurin B1 were shown to develop DM due to insufficient insulin production, while transgenic expression of constitutively active NFAT protected against DM [39]. In another study on immunosuppressants, rapamycin inhibited the mammalian target of rapamycin without affecting calcineurin activity, and also inhibited glucose-stimulated insulin secretion (GSIS) in β-cells [40,41]. Although the therapeutic ranges of Tac and rapamycin are comparable, the potency of rapamycin in inhibiting insulin secretion seemed to be much lower than that of Tac in our study [29]. This may partly explain why rapamycin has less diabetogenic activity. However, the role of rapamycin in PTDM is still under investigation [17,42]. Recently, the suppression of carbohydrate metabolism by rapamycin was suggested as a possible mechanism for the inhibition of insulin secretion [43]. However, the mechanism of action of rapamycin in the insulin secretory pathway is largely unknown.
Diabetes is caused by an imbalance in insulin production and peripheral insulin sensitivity. Despite some controversy, insulin secretory dysfunction is thought to be the dominant mechanism for the development of PTDM. We found that β-cell dysfunction plays a major role in the development of PTDM, which is consistent with previous reports [15,21]. On the issue of 'Diabetes Care' [15], we enrolled a total of 77 subjects with normal glucose tolerance (NGT), and an oral glucose tolerance test (OGTT) was performed 1 week before transplantation and repeated at 1 and 7 years after transplantation [15]. The overall incidence of PTDM was 39% at 1 year and 35.1% at 7 years post-transplantation.
The following conclusions were drawn based on the results of these studies: first, the fasting and 2-hour plasma glucose levels before transplantation in the IGT and PTDM after transplantation groups were significantly higher than those in the NGT after transplantation group. Second, patients with decreased insulin-secretory capacity, rather than decreased insulin sensitivity, were predisposed to developing PTDM. Compared with values before transplantation, the indices of insulin secretion and insulin sensitivity deteriorated at 1 year after transplantation. The insulin sensitivity index (ISI), representing insulin sensitivity, was not statistically different among groups after transplantation. However, the area under the curve of insulin on OGTT, representing insulin secretory function, was significantly lower in the PTDM group than in the NGT group. Proinsulin and proinsulin/insulin ratios have been proposed as indices for reduced maximum β-cell secretory capacity in patients with type 1 and type 2 diabetes [44,45]. Maximum β-cell secretory capacity plays a pivotal role in the response to glucose loading, which is associated with deterioration in glucose tolerance. In our study, proinsulin/insulin ratios in the IGT and PTDM groups were significantly higher than in the NGT group at both pre- and post-transplantation periods. Taken together, this indicates that β-cell dysfunction was a more important factor for the development of PTDM.
PTDM may reflect a genetic predisposition to diabetes influenced by multiple environmental factors. Twenty-six percent of patients with a family history of diabetes develop PTDM, while only 14% of patients without a family history of diabetes develop PTDM [46]. This finding suggests that genetic variants typically associated with T2D are also likely associated with PTDM. However, only a few of the genetic polymorphisms associated with T2D have been studied in detail in transplant patients. Some studies have shown a significant association between PTDM and genetic polymorphisms. These polymorphisms were associated with genes involving insulin secretion (KCNJ11, HNF4A, NFATc4, ENPP1/PC-1 [K121Q], CCL5, VDR) [47-52], insulin resistance (adiponectin, adiponectin receptor 1, PAI-1) [53-55], and oxidative stress (glutathione S-transferase gene, SOD) [56,57]. One functional study and our three genetic association studies comprising a renal allograft cohort found that TCF7L2, a T2D susceptibility gene, led to a two-fold increase in the risk of T2D. In addition, we found that the TCF7L2 rs7903146 genetic variation is also significantly associated with an increased risk of developing PTDM in kidney transplant recipients [58]. Zinc found in the zinc transporter-8 (ZnT-8) gene is involved in modulating insulin secretion. The R325W (rs13266634) non-synonymous polymorphism in the islet-specific zinc transporter protein gene SLC30A8 is associated with T2D and also may be associated with a defect in insulin secretion. To investigate the association between genetic variations in the SLC30A8 gene and PTDM in renal-transplant recipients [59], we recruited a total of 624 renal-allograft recipients without a documented history of diabetes. The prevalence of PTDM was 33.8% in patients with the R/R genotype, 26.8% in those with the R/W genotype, and 19.8% in those with the W/W genotype. In addition, a strong association between the number of W alleles and PTDM risk reduction (P=0.007) was found. Patients with at least one W allele had a lower risk of PTDM compared to those with the R/R genotype (R/W RR, 0.78, P=0.126; W/W RR, 0.52, P=0.007). The effect of the SLC30A8 genotype remained significant even after adjusting for age, gender, body weight gain, and type of immunosuppressant (R/W hazard ratio [HR], 0.77, P=0.114; W/W HR, 0.58, P=0.026). Therefore the SLC30A8 rs13266634 gene variation appears to be associated with protection against the development of PTDM in renal-allograft recipients.
There is an association between PTDM and 17 single nucleotide polymorphisms (SNPs) located within 15 genes. Genome-wide association studies determined that these genes were related to diabetes susceptibility [60]. We found an association between the development of PTDM and the following SNPs: HHEX (rs1111875, rs7923837, and rs5015480), CDKAL1 rs10946398, CDKN2A/B rs10811661, IGF2BP2 rs4402960, FTO rs8050136, WFS1 rs734312, JAZF1 rs864745, CDC123/CAMK1D rs12779790, TSPAN8 rs7961581, THADA rs7578597, ADAMTS9 rs4607103, NOTCH2 rs1092391, and KCNQ1 rs2237892. Six loci were significantly associated with PTDM development: HHEX rs1111875 (odds ratio [OR], 1.47; P=0.007), HHEX rs7923837 (OR, 2.32; P=0.014), HHEX rs5015480 (OR, 1.59; P=0.003), CDKAL1 rs10946398 (OR, 1.43; P=0.008), CDKN2A/B rs10811661 (OR, 1.33; P=0.039), and KCNQ1 rs2237892 (OR, 1.46; P=0.009).
Recently, we investigated the functional aspect of the zinc transporter gene, based on the hypothesis that the polymorphic residue at position 325 of ZnT-8 might determine susceptibility to CsA suppression of insulin secretion [29]. INS-1E cells expressing the W325 variant showed enhanced GSIS and were less sensitive to CsA suppression of GSIS. A reduced number of insulin granule fusion events accompanied the decrease in insulin secretion in CsA-treated cells expressing ZnT-8 R325; however, ZnT-8 W325-expressing cells exhibited resistance to the dampening of insulin granule fusion by CsA and transported zinc ions into secretory vesicles more efficiently. Both Tac and rapamycin caused similar suppression of GSIS in cells expressing ZnT-8 R325. However, cells expressing ZnT-8 W325 were resistant to Tac, but not to rapamycin. Overexpression of the Down's syndrome candidate region-1 (DSCR1), an endogenous calcineurin inhibitor, and subsequent calcineurin inhibition significantly reduced GSIS in cells expressing the R325 but not the W325 variant, suggesting that differing susceptibility to CsA may be due to different interactions with calcineurin. These data suggest that the ZnT-8 W325 variant is protective against CsA-induced suppression of insulin secretion. Tolerance of ZnT-8 W325 to calcineurin activity may account for its protective effect in PTDM.
DM is a serious and frequent complication of renal transplantation. Although DM ultimately results from decreased insulin secretion and increased insulin resistance, our data suggest that β-cell dysfunction, rather than insulin resistance, may be the main contributing factor in the development of PTDM. PTDM is not always permanent and may resolve within weeks or months, sometimes without treatment. The aforementioned risk factors seem to be related to the different courses of PTDM. The ability to predict a patient's risk for developing PTDM would be of considerable benefit in selecting appropriate immunosuppressive regimens for individuals.
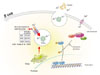
Genotyping diabetes-related polymorphisms is one possible method for predicting a patient's risk for developing PTDM. Our data suggest that genetic variations such as TCF7L2, SLC30A8, HHEX, CDKAL1, CDKN2A/B, and KCNQ1 are associated with PTDM in Korea. Notably, SLC30A8 encodes for β-cell-specific ZnT-8. The same allelic variant (which is a substitution of tryptophan for arginine at residue 325) of ZnT-8 is also associated with reduced incidence of PTDM in renal allograft recipients (Fig. 1). A single nucleotide substitution in SLC30A8 confers resistance to CsA-induced β-cell dysfunction by altering susceptibility to calcineurin inhibition. Our data suggest that ZnT-8 might be an important molecule connecting calcineurin inhibition by CsA to downregulation of β-cell insulin secretion, and this signaling pathway might be a new therapeutic target for the treatment of CsA-induced β-cell complications that occur after organ transplantation.
Figures and Tables
Fig. 1
Schematic representation of vesicular zinc transporter-8 (ZnT-8) expression and R325W polymorphism in pancreatic islet β-cells. The single-nucleotide polymorphism rs13266634 of SLC30A8 is associated with postrenal transplantation diabetes mellitus (PTDM) in renal allograft recipients, as demonstrated by Kang et al. [59]. SLC30A8 encodes ZnT-8, which is specifically expressed in β-cells. ZnT-8 promotes zinc accumulation in secretory vesicles, and vesicular zinc is important for the formation of the zinc-insulin hexamer. Calcineurin is the intracellular target of the immunosuppressive drugs CsA and tacrolimus, and calcineurin activity is known to play a role in insulin gene transcription through the regulation of NFATc activity (Reproduced from Kim I, et al. Pharmacogenomics J 2011;11:191-8, with permission from Nature Publishing Group, a division of Macmillan Publishers Limited) [29].
References
1. Miles AM, Sumrani N, Horowitz R, Homel P, Maursky V, Markell MS, Distant DA, Hong JH, Sommer BG, Friedman EA. Diabetes mellitus after renal transplantation: as deleterious as non-transplant-associated diabetes? Transplantation. 1998. 65:380–384.
2. Bloom RD, Crutchlow MF. New-onset diabetes mellitus in the kidney recipient: diagnosis and management strategies. Clin J Am Soc Nephrol. 2008. 3:Suppl 2. S38–S48.
3. Starzl TE, Marchioro TL, Rifkind D, Holmes JH, Rowlands DT Jr, Waddell WR. Factors in successful renal transplantation. Surgery. 1964. 56:296–318.
4. Crutchlow MF, Bloom RD. Transplant-associated hyperglycemia: a new look at an old problem. Clin J Am Soc Nephrol. 2007. 2:343–355.
5. Hill CM, Douglas JF, Rajkumar KV, McEvoy J, McGeown MG. Glycosuria and hyperglycaemia after kidney transplantation. Lancet. 1974. 2:490–492.
6. Arner P, Gunnarsson R, Blomdahl S, Groth CG. Some characteristics of steroid diabetes: a study in renal-transplant recipients receiving high-dose corticosteroid therapy. Diabetes Care. 1983. 6:23–25.
7. Rao M, Jacob CK, Shastry JC. Post-renal transplant diabetes mellitus: a retrospective study. Nephrol Dial Transplant. 1992. 7:1039–1042.
8. Ahn KJ, Kim YS, Lee HC, Park K, Huh KB. Clinical characteristics and possible risk factors in postrenal transplant diabetes mellitus. Transplant Proc. 1992. 24:1581–1582.
9. Basri N, Aman H, Adiku W, Baraqdar A, Bonatero I, Nezamuddin N. Diabetes mellitus after renal transplantation. Transplant Proc. 1992. 24:1780–1781.
10. Sumrani NB, Delaney V, Ding ZK, Davis R, Daskalakis P, Friedman EA, Butt KM, Hong JH. Diabetes mellitus after renal transplantation in the cyclosporine era: an analysis of risk factors. Transplantation. 1991. 51:343–347.
11. von Kiparski A, Frei D, Uhlschmid G, Largiader F, Binswanger U. Post-transplant diabetes mellitus in renal allograft recipients: a matched-pair control study. Nephrol Dial Transplant. 1990. 5:220–225.
12. Lee HC, Nam MS, Nam SY, Cha BS, Lee JH, Song YD, Lee EJ, Lim SK, Kim KR, Kim YS, Park K, Huh KB. Posttransplant diabetes mellitus after renal transplantation in Korea. Transplant Proc. 1996. 28:1159–1160.
13. Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, Woodworth TG, Brennan DC. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant. 2003. 3:590–598.
14. Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003. 3:178–185.
15. Hur KY, Kim MS, Kim YS, Kang ES, Nam JH, Kim SH, Nam CM, Ahn CW, Cha BS, Kim SI, Lee HC. Risk factors associated with the onset and progression of posttransplantation diabetes in renal allograft recipients. Diabetes Care. 2007. 30:609–615.
16. Hjelmesaeth J, Hartmann A, Kofstad J, Stenstrom J, Leivestad T, Egeland T, Fauchald P. Glucose intolerance after renal transplantation depends upon prednisolone dose and recipient age. Transplantation. 1997. 64:979–983.
17. Rodrigo E, Fernandez-Fresnedo G, Valero R, Ruiz JC, Pinera C, Palomar R, Gonzalez-Cotorruelo J, Gomez-Alamillo C, Arias M. New-onset diabetes after kidney transplantation: risk factors. J Am Soc Nephrol. 2006. 17:12 Suppl 3. S291–S295.
18. Markell M. New-onset diabetes mellitus in transplant patients: pathogenesis, complications, and management. Am J Kidney Dis. 2004. 43:953–965.
19. Lanerolle RD, de Abrew K, Fernando DJ, Sheriff MH. Post-renal transplant diabetes in Sri Lanka. Transplant Proc. 1996. 28:1945–1947.
20. Bloom RD, Rao V, Weng F, Grossman RA, Cohen D, Mange KC. Association of hepatitis C with posttransplant diabetes in renal transplant patients on tacrolimus. J Am Soc Nephrol. 2002. 13:1374–1380.
21. Nam JH, Mun JI, Kim SI, Kang SW, Choi KH, Park K, Ahn CW, Cha BS, Song YD, Lim SK, Kim KR, Lee HC, Huh KB. beta-Cell dysfunction rather than insulin resistance is the main contributing factor for the development of postrenal transplantation diabetes mellitus. Transplantation. 2001. 71:1417–1423.
22. Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 2004. 4:583–595.
23. Ekstrand AV, Eriksson JG, Gronhagen-Riska C, Ahonen PJ, Groop LC. Insulin resistance and insulin deficiency in the pathogenesis of posttransplantation diabetes in man. Transplantation. 1992. 53:563–569.
24. Midtvedt K, Hartmann A, Hjelmesaeth J, Lund K, Bjerkely BL. Insulin resistance is a common denominator of post-transplant diabetes mellitus and impaired glucose tolerance in renal transplant recipients. Nephrol Dial Transplant. 1998. 13:427–431.
25. Shimizu M, Iino Y, Terashi A. Improvement of insulin sensitivity after renal transplantation measured by a glucose clamp technique. Nihon Ika Daigaku Zasshi. 1998. 65:50–54.
26. Hagen M, Hjelmesaeth J, Jenssen T, Morkrid L, Hartmann A. A 6-year prospective study on new onset diabetes mellitus, insulin release and insulin sensitivity in renal transplant recipients. Nephrol Dial Transplant. 2003. 18:2154–2159.
27. Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of Korean type 2 diabetes mellitus. Metabolism. 2001. 50:590–593.
28. Rhee SY, Woo JT. The prediabetic period: review of clinical aspects. Diabetes Metab J. 2011. 35:107–116.
29. Kim I, Kang ES, Yim YS, Ko SJ, Jeong SH, Rim JH, Kim YS, Ahn CW, Cha BS, Lee HC, Kim CH. A low-risk ZnT-8 allele (W325) for post-transplantation diabetes mellitus is protective against cyclosporin A-induced impairment of insulin secretion. Pharmacogenomics J. 2011. 11:191–198.
30. Nielsen JH, Mandrup-Poulsen T, Nerup J. Direct effects of cyclosporin A on human pancreatic beta-cells. Diabetes. 1986. 35:1049–1052.
31. Robertson RP. Cyclosporin-induced inhibition of insulin secretion in isolated rat islets and HIT cells. Diabetes. 1986. 35:1016–1019.
32. Paty BW, Harmon JS, Marsh CL, Robertson RP. Inhibitory effects of immunosuppressive drugs on insulin secretion from HIT-T15 cells and Wistar rat islets. Transplantation. 2002. 73:353–357.
33. Polastri L, Galbiati F, Bertuzzi F, Fiorina P, Nano R, Gregori S, Aldrighetti L, Pozza G, Secchi A, Adorini L, Davalli AM. Secretory defects induced by immunosuppressive agents on human pancreatic beta-cells. Acta Diabetol. 2002. 39:229–233.
34. Redmon JB, Olson LK, Armstrong MB, Greene MJ, Robertson RP. Effects of tacrolimus (FK506) on human insulin gene expression, insulin mRNA levels, and insulin secretion in HIT-T15 cells. J Clin Invest. 1996. 98:2786–2793.
35. Uchizono Y, Iwase M, Nakamura U, Sasaki N, Goto D, Iida M. Tacrolimus impairment of insulin secretion in isolated rat islets occurs at multiple distal sites in stimulus-secretion coupling. Endocrinology. 2004. 145:2264–2272.
36. Lawrence MC, Bhatt HS, Watterson JM, Easom RA. Regulation of insulin gene transcription by a Ca(2+)-responsive pathway involving calcineurin and nuclear factor of activated T cells. Mol Endocrinol. 2001. 15:1758–1767.
37. Oetjen E, Baun D, Beimesche S, Krause D, Cierny I, Blume R, Dickel C, Wehner S, Knepel W. Inhibition of human insulin gene transcription by the immunosuppressive drugs cyclosporin A and tacrolimus in primary, mature islets of transgenic mice. Mol Pharmacol. 2003. 63:1289–1295.
38. Lawrence MC, Bhatt HS, Easom RA. NFAT regulates insulin gene promoter activity in response to synergistic pathways induced by glucose and glucagon-like peptide-1. Diabetes. 2002. 51:691–698.
39. Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, Kim SK. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006. 443:345–349.
40. Zhang N, Su D, Qu S, Tse T, Bottino R, Balamurugan AN, Xu J, Bromberg JS, Dong HH. Sirolimus is associated with reduced islet engraftment and impaired beta-cell function. Diabetes. 2006. 55:2429–2436.
41. Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, Berthault MF, Magnan C, Cerasi E, Kaiser N, Leibowitz G. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008. 57:945–957.
42. Veroux M, Corona D, Giuffrida G, Gagliano M, Sorbello M, Virgilio C, Tallarita T, Zerbo D, Giaquinta A, Fiamingo P, Macarone M, Li Volti G, Caglia P, Veroux P. New-onset diabetes mellitus after kidney transplantation: the role of immunosuppression. Transplant Proc. 2008. 40:1885–1887.
43. Shimodahira M, Fujimoto S, Mukai E, Nakamura Y, Nishi Y, Sasaki M, Sato Y, Sato H, Hosokawa M, Nagashima K, Seino Y, Inagaki N. Rapamycin impairs metabolism-secretion coupling in rat pancreatic islets by suppressing carbohydrate metabolism. J Endocrinol. 2010. 204:37–46.
44. Heaton DA, Millward BA, Gray IP, Tun Y, Hales CN, Pyke DA, Leslie RD. Increased proinsulin levels as an early indicator of B-cell dysfunction in non-diabetic twins of type 1 (insulin-dependent) diabetic patients. Diabetologia. 1988. 31:182–184.
45. Roder ME, Porte D Jr, Schwartz RS, Kahn SE. Disproportionately elevated proinsulin levels reflect the degree of impaired B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998. 83:604–608.
46. Sumrani N, Delaney V, Ding Z, Davis R, Daskalakis P, Friedman EA, Butt KM, Hong JH. Posttransplant diabetes mellitus in cyclosporine-treated renal transplant recipients. Transplant Proc. 1991. 23(1 Pt 2):1249–1250.
47. Tavira B, Coto E, Torres A, Diaz-Corte C, Diaz-Molina B, Ortega F, Arias M, Diaz JM, Selgas R, Lopez-Larrea C, Ruiz-Ortega M, Ortiz A, Gonzalez E, Campistol JM, Alvarez V. The Pharmacogenetics of tacrolimus REDINREN study group. Association between a common KCNJ11 polymorphism (rs5219) and new-onset posttransplant diabetes in patients treated with Tacrolimus. Mol Genet Metab. 2012. 105:525–527.
48. Yang J, Hutchinson II, Shah T, Min DI. Genetic and clinical risk factors of new-onset diabetes after transplantation in Hispanic kidney transplant recipients. Transplantation. 2011. 91:1114–1119.
49. Chen Y, Sampaio MS, Yang JW, Min D, Hutchinson IV. Genetic polymorphisms of the transcription factor NFATc4 and development of new-onset diabetes after transplantation in Hispanic kidney transplant recipients. Transplantation. 2012. 93:325–330.
50. Szuszkiewicz M, Bell J, Vazquez M, Adams-Huet B, Grundy SM, Chandalia M, Abate N. ENPP1/PC-1 K121Q and other predictors of posttransplant diabetes. Metab Syndr Relat Disord. 2011. 9:25–29.
51. Jeong KH, Moon JY, Chung JH, Kim YH, Lee TW. Significant associations between CCL5 gene polymorphisms and post-transplantational diabetes mellitus in Korean renal allograft recipients. Am J Nephrol. 2010. 32:356–361.
52. Numakura K, Satoh S, Tsuchiya N, Horikawa Y, Inoue T, Kakinuma H, Matsuura S, Saito M, Tada H, Suzuki T, Habuchi T. Clinical and genetic risk factors for posttransplant diabetes mellitus in adult renal transplant recipients treated with tacrolimus. Transplantation. 2005. 80:1419–1424.
53. Kang ES, Magkos F, Kim BS, Zhai R, Su L, Kim YS, Christiani DC, Lee HC, Mantzoros CS. Variants of the adiponectin and adiponectin receptor-1 genes and posttransplantation diabetes mellitus in renal allograft recipients. J Clin Endocrinol Metab. 2012. 97:E129–E135.
54. Yu SJ, Peng L, Xie XB, Peng FH, Fang CH, Wang Y, Lan GB. Correlation between HLA and posttransplantation diabetes mellitus in the Han population in South China. Transplant Proc. 2010. 42:2509–2512.
55. Chang HR, Yang SF, Tsai JP, Hsieh MC, Wu SW, Tsai HC, Hung TW, Huang JH, Lian JD. Plasminogen activator inhibitor-1 5G/5G genotype is a protecting factor preventing posttransplant diabetes mellitus. Clin Chim Acta. 2011. 412:322–326.
56. Tsai JP, Yang SF, Wu SW, Hung TW, Tsai HC, Lian JD, Chang HR. Glutathione S-transferase gene polymorphisms are not major risks for susceptibility to posttransplantation diabetes mellitus in Taiwan renal transplant recipients. J Clin Lab Anal. 2011. 25:432–435.
57. Dutkiewicz G, Domanski L, Pawlik A, Binczak-Kuleta A, Safranow K, Ciechanowicz A, Dziedziejko V, Pietrzak-Nowacka M, Ciechanowski K. Polymorphisms of superoxide dismutase, glutathione peroxidase and catalase genes in patients with post-transplant diabetes mellitus. Arch Med Res. 2010. 41:350–355.
58. Kang ES, Kim MS, Kim YS, Hur KY, Han SJ, Nam CM, Ahn CW, Cha BS, Kim SI, Lee HC. A variant of the transcription factor 7-like 2 (TCF7L2) gene and the risk of posttransplantation diabetes mellitus in renal allograft recipients. Diabetes Care. 2008. 31:63–68.
59. Kang ES, Kim MS, Kim YS, Kim CH, Han SJ, Chun SW, Hur KY, Nam CM, Ahn CW, Cha BS, Kim SI, Lee HC. A polymorphism in the zinc transporter gene SLC30A8 confers resistance against posttransplantation diabetes mellitus in renal allograft recipients. Diabetes. 2008. 57:1043–1047.
60. Kang ES, Kim MS, Kim CH, Nam CM, Han SJ, Hur KY, Ahn CW, Cha BS, Kim SI, Lee HC, Kim YS. Association of common type 2 diabetes risk gene variants and posttransplantation diabetes mellitus in renal allograft recipients in Korea. Transplantation. 2009. 88:693–698.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download