Abstract
Background
We examined the change in the levels of incretin hormone and effects of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) on insulin secretion in women with previous gestational diabetes (pGDM).
Methods
A 75-g oral glucose tolerance test (OGTT) was conducted on 34 women with pGDM. In addition, 11 women with normal glucose tolerance, matched for age, height and weight, were also tested. The insulin, GIP, GLP-1, and glucagon concentrations were measured, and their anthropometric and biochemical markers were also measured.
Results
Among 34 women with pGDM, 18 had normal glucose tolerance, 13 had impaired glucose tolerance (IGT) and 1 had diabetes. No significant differences were found in GLP-1 concentration between the pGDM and control group. However, a significantly high level of glucagon was present in the pGDM group at 30 minutes into the OGTT. The GIP concentration was elevated at 30 minutes and 60 minutes in the pGDM group. With the exception of the 30-minute timepoint, women with IGT had significantly high blood glucose from 0 to 120 minutes. However, there was no significant difference in insulin or GLP-1 concentration. The GIP level was significantly high from 0 to 90 minutes in patients diagnosed with IGT.
Conclusion
GLP-1 secretion does not differ between pGDM patients and normal women. GIP was elevated, but that does not seem to induce in increase in insulin secretion. Therefore, we conclude that other factors such as heredity and environment play important roles in the development of type 2 diabetes.
Gestational diabetes mellitus (GDM) is defined as glucose intolerance of variable severity with onset or first recognition during pregnancy [1]. The prevalence of GDM is reported as ~14% of all pregnancies. Since gestational diabetes causes an increase in problems such as macrosomia, birth trauma, neonatal hypoglycemia, and obstetrical complications such as Cesarean delivery and preeclampsia, it has been considered as one of major metabolic disorders during pregnancy [2]. Additionally, although the glucose tolerance of most women with GDM returns to normal, women with GDM are at an increased risk for type 2 diabetes mellitus later in life [2]. In regards to attempting to understand the pathogenesis of type 2 diabetes in women with GDM, studies for insulin secretion and insulin sensitivity in women with previous GDM have been performed. When Korean women with previous GDM were measured for insulin secretion and insulin sensitivity, even though normal glucose tolerance was maintained after delivery in women with GDM, but a significant decrease in their insulin secretion was reported compared to women without GDM [3,4].
There is an interaction called the enteroinsular axis, which occurs between the intestine and the endocrine pancreas and bridges food intake and secretion of pancreatic hormones through incretin hormones [5]. Due to these hormones, we know that nutrients that are liberated in the intestines as a result of oral food consumption control the secretion of pancreatic hormone secretion (stimulate insulin secretion). The insulin-stimulating effect of the oral glucose load from incretin was found to be much larger than the insulin-stimulating effect of the intravenous glucose tolerance test [6]. Glucose-dependent insulinotropic polypeptide (GIP) and glucagon like pepide1 (GLP-1) are among the well-known incretin hormones. The concentrations of these hormones were reported to be decreased or the stimulating effect of insulin secretion was reduced in type 2 diabetes, and a 50% reduction of these hormones in the sisters of the diabetes patients was reported [7-9]. The decreasing incretin effect of insulin secretion in type 2 diabetes was known, while the one of main pathogenesis pathway of type 2 diabetes, insulin secretion impairment is sometimes assumed to be an incretin disorder [8,10]. In terms of insulin resistance and insulin secretion disorders, in gestational diabetes, that is similar with type 2 diabetes, the likelihood of the failure of incretin effect has been reported. However, using women with gestational diabetes as the primary subjects for a study on incretin hormone is considered to be controversial [10,11].
To find a relationship between insulin secretion capacity and concentration of incretin hormones in Korean women with GDM, we performed an oral glucose tolerance test (OGTT), and measured the concentration of GLP-1, GIP, and glucagon during OGTT.
We recruited women who diagnosed with GDM by NDDG criteria from January of 2000 to February of 2003. Thirty four volunteers, who had a normal glucose tolerance at 2 months postpartum 75-g OGTT, participated in this study. The diagnostic procedure and criteria for GDM and diabetes were described in great detail in previous studies [12,13].
We also recruited 11 healthy women with no history of GDM as a normal control group based on their age, height, and weight. They had no history of diabetes, and no family history of diabetes. All study subjects did not have anemia (hemoglobin<11 g/dL), liver dysfunction (2-fold increased liver enzyme values), or plasma creatinine elevation (>1.2 mg/dL).
The weight, height, and waist circumference were measured in a standardized way for all subjects. When measurements were collected, subjects were required to remove their shoes and wear light clothing. Using a mercury sphygmomanometer, the blood pressure was measured three times with a five-minute rest between measurements while subjects were in a seated position after a 10-minute period. The waist circumference was measured at the narrowest point between the lower border of the ribs and the iliac crest. The 75-g OGTT was performed in the morning after 14 hours of fasting. After sampling venous blood, glucose solution (Glucola) was consumed by subjects within 5 minutes, and then venous blood samples were collected at 30, 60, 90, and 120 minutes. Plasma glucose concentrations were measured using the glucose oxidase method via the YSI 2300-STAT glucose analyzer (Yellow Spring Instrument Co., Yellow Springs, OH, USA). Using a commercial kit (Linco Research, St. Louis, MO, USA), the plasma insulin concentrations were measured using a radioimmunoassay.
For the purpose of measuring GIP, GLP-1, and glucagon concentrations, aprotinin (Trasylol; 20,000 Kallikrein Inhibitor Units/mL, 200 µL per 10 mL blood; Bayer, Leverkusen, Germany) was added to EDTA tubes. The tubes were placed in ice-containing coolers, and then after venous blood sampling, they were centrifuged at 4℃ and stored in a freezer at -72℃. GIP and glucagon were measured using a commercially available enzyme immunoassay kit (Phoenix Pharmaceuticals, Belmont, CA, USA), and GLP-1 measurements were taken using a kit that was capable of measuring GLP-1 (7-36) (Phoenix Pharmaceuticals). The intra-assay coefficient of variation of GIP, GLP-1, and glucagon was 5%, 7%, and 4.8%, respectively, and the inter-assay coefficient of variation was 14%, 8%, and 12%, respectively.
The insulin resistance index was based on homeostasis model assessment-insulin resistance (HOMA-IR) [14]. HOMA-IR was calculated using the following formula: fasting plasma glucose (mmol/L)×fasting insulin (mU/L)/22.5. Total cholesterol, triglycerides, and high density lipoprotein (HDL) cholesterol were measured using an automatic analyzer (Hitachi 747; Hitachi, Tokyo, Japan). Insulin secretion capacity was evaluated using the HOMA-beta and insulinogenic index (30 minutes) [14,15]. The SPSS program version 15.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and the results were expressed as mean±standard deviation. Discrete variables were analyzed using Kolmogorov-Smirnov analysis through non-parametric methods, and continuous variables were analyzed using ANOVA. Results with a P value less than 0.05 were considered to be statistically significant.
According to the criteria of the American Diabetes Association, our results from the 75-g OGTT in women with GDM revealed 1 woman with diabetes, 13 with impaired fasting glucose or impaired glucose tolerance, and 18 with normal glucose tolerance, and all subjects of the control group were confirmed to have normal glucose tolerance as well. There was no difference in the age, height, body weight, body mass index, and blood pressure between the GDM group and the normal control group. The concentration of A1C was higher in the GDM group (5.3±0.2% vs. 5.0±0.3%, P=0.02). The total cholesterol, triglyceride, low density lipoprotein (LDL), and HDL concentrations in the plasma in both groups were not significantly different (Table 1).
There was no difference in the fasting glucose levels between the GDM group and the normal group (99.1±20.4 mg/dL vs. 96.3±11.6 mg/dL, respectively, P=0.572). The glucose concentration during OGTT in the GDM group was significantly higher than that in the normal group at 60 minutes (163.8±33.3 mg/dL vs. 133.1±28.8 mg/dL, P=0.009), at 90 minutes (144.7±37.7 mg/dL vs. 119.0±24.7 mg/dL, P=0.043), and at 120 minutes (130.7±28.0 mg/dL vs. 116.2±15.4 mg/dL, respectively, P=0.041). The insulin concentration in the GDM group peaked at 60 minutes, and then showed a pattern of gradual decrease. The concentrations of insulin in the subjects from the normal control group at 60 minutes and 90 minutes were lost, and thus, they could not be measured. However, the insulin concentration of the two groups that were available for measurement showed no significant difference.
For the incretin hormone levels measured at 30-minute intervals during OGTT, there was no significant difference in GLP-1 concentrations between the GDM group and the normal control group. GIP concentrations were significantly higher in the GDM group at 0 minute, and at 60 minutes (Fig. 1). Although the concentration of GLP-1 in the gestational diabetes group during the OGTT showed no significant increase, the concentration of GLP-1 in the control group from 0 minute (43.6±25.9 ng/mL) to 30 minutes (59.8±29.4 ng/mL) showed a tendency to increase (P=0.064), and showed a significant increase at 60 minutes (60.3±31.7 ng/mL, P=0.033), and at 120 minutes (61.0±30.4 ng/mL, P=0.013). Although there was no significant increase in the GIP concentration in the GDM group, the GIP concentration in the normal group was 28.9±12.1 ng/mL at 0 minutes, and it increased to 37.0±9.6 ng/mL (P=0.002) at 30 minutes, and 38.9±14.0 ng/mL (P=0.000) at 90 minutes.
There was not a significant difference in the glucagon concentration during the OGTT, when compared between two groups, but glucagon concentration at 30 minutes was higher in the GDM group (40.8±8.7 pg/mL vs. 34.9±3.6 pg/mL, P=0.037). However, after adjustment for age (general linear model), the GLP-1 and glucagon concentrations were not significantly different, and GIP concentration at 0 minute was significantly elevated in the GDM group.
When we compared subject with GDM who were diagnosed with impaired glucose tolerance with individuals in the normal control group, the plasma glucose concentrations during OGTT except the 30-minute mark, were significantly higher in the GDM with impaired glucose tolerance group (Fig. 2). However, the plasma insulin levels were similar between two groups. The GLP-1 concentrations during OGTT did not show a difference over time, but the GIP concentrations of the GDM with impaired glucose tolerance group were significantly higher between 0 and 90 minutes. The glucagon concentration at 30 minutes was higher in the GDM with impaired glucose tolerance group (40.2±6.8 pg/mL vs. 34.9±3.6 pg/mL, P=0.032).
When the insulin resistance of the GDM group and the normal control group were compared using HOMA-IR, there was no significant difference, but the insulin secretion capacity measured by the insulinogenic index was significantly low in the GDM group, but there was no significant difference in the HOMA-beta (Table 2). When the incretin hormone secretion during the OGTT was compared using the area under the curve (AUC), the AUC_GLP-10-2hr and AUC_GIP0-2hr in the GDM group and the normal control groups did not differ, and AUC_Glucagon0-2hr was higher in the GDM group than in the normal control group. Additionally, when the blood glucose levels and the incretin hormone concentrations over-time-correlation were analyzed, the GIP_30min and the 30-minute plasma glucose (r=0.429, P=0.004) had a positive correlation. However, there was also no significant correlation between blood glucose and GLP-1 and glucagon concentrations.
In this study, the glucose concentration at 60-120 minutes during the OGTT was higher in the GDM group, but there was no difference in the insulin concentrations during the OGTT between the GDM and normal control group. However, when insulin secretion capacity using the insulinogenic index was compared, the insulin secretion in the GDM group was lower, and there was no difference in the insulin resistance measured using HOMA-IR. Through our findings that were consistent with those of previous studies, we confirmed that the impairment of insulin secretion is the main clinical characteristic in women with GDM [3,4,12].
In the patients with type 2 diabetes, the decreased effect of GLP-1 and GIP in the insulin secretion are reported in the several studies that we reviewed [7,8]. Therefore, incretin hormone decrease or impairment appears to be the main mechanism underlying insulin secretion disorder in type 2 diabetes. However, the studies in women with GDM, who are at high risk for type 2 diabetes, on GLP-1 and GIP are limited, and the results have been inconsistent. In women with GDM, during the initial 30 minutes of an OGTT, GLP-1 secretion was reported to be significantly low, while there was no difference reported in the another studies [10,11,16].
In this study, during the OGTT, there was no significant difference in the GLP-1 concentration between women with GDM and normal females. Additionally, the measured GIP concentration in women with GDM was higher than expected. These results were consistent with the finding that when Cypryk et al. [10] measured the GLP-1 concentration during the OGTT in females with GDM, the result was the same as that for normal females, and there was also no difference in the GIP concentration. Even in studies involving the immediate family members of patients with type 2 diabetes or individuals with impaired glucose tolerance, there was no difference in the GLP concentration during the OGTT, and in some cases, it was reported that the GIP value were either similar or were higher compared with that of normal control [17,18]. However, in one study, there was no significant change of GLP-1 concentration in the OGTT, and early (at 30 minutes) GLP-1 concentration revealed a decrease of approximately 20% in the GDM group [11]. The different findings among studies in women with GDM might be due to different clinical characteristics such as ethnicity, degree of obesity, and relative risk of developing diabetes.
In this study, at fasting and 60 minutes after glucose load the measured GIP concentration in subjects with GDM was higher than the normal control. This finding suggests that the GIP effect on the insulin secretion is reduced in GDM patients. When GDM women with impaired glucose tolerance were analyzed separately, the significant increase of the GIP concentration from 0 minutes to 90 minutes during the OGTT was also founded. Between the GDM group and control group, there was no difference in the fasting plasma glucose, but the increase in the GIP concentration in the GDM group was made apparent. This finding was consistent with animal knockout model. In GLP-1 receptor knockout mice, fasting glucose was increased and insulin secretion by the glucose load was decreased. However, there was no difference in fasting glucose levels, but insulin secretion by the glucose load was decreased in the GIP receptor knockout mice [19]. In cases where diabetes occurs in women with GDM, it can be assumed that the effect of GIP's insulin stimulation capacity is more impaired than that of GLP-1. Additionally, despite the absence of change in the concentration of GLP-1, at 30 minutes after the glucose load, glucagon concentration in the GDM group, and GDM with impaired glucose tolerance group was higher than normal control group. Inappropriate suppression of glucagon level was considered to be related to the increase in the glucose concentration.
A limitation of this study was that the GLP-1 concentration in the GDM group did not rise significantly during the initial phase of the OGTT. However, GLP-1 and GIP concentrations during the OGTT increased significantly in the normal control group. In comparison to the control group, the stimulation of incretin hormone secretion in the GDM group by rising glucose levels might be weak. So, there might be defect in incretin hormone secretion pathway or receptor resistance in women with GDM. In addition, the patient groups and the control groups did not significantly differ in age, but the control group had limited information because only small individuals had a baby.
In conclusion, GLP-1 concentration was not different between women with GDM and healthy women, and GIP concentration in women with GDM was higher than the normal control, but there was no visible effect on insulin secretion. The development of diabetes in women with GDM appears to be due to impaired beta cell function that is not related to incretin effect.
Figures and Tables
Fig. 1
Plasma concentrations of glucose (A), insulin (B), glucagon like pepide1 (GLP-1) (C), glucose-dependent insulinotropic polypeptide (GIP) (D), and glucagon (E) after the ingestion of 75-g oral glucose in 34 women with a history of previous gestational diabetes mellitus (pGDM) (square symbols) and 11 control women (round symbols). Data are presented as means±standard deviation; P values were calculated using repeated measures ANOVA. aSignificant differences at individual time points (P<0.05 by one-way ANOVA).

Fig. 2
Plasma concentrations of glucose (A), insulin (B), glucagon like pepide1 (GLP-1) (C), glucose-dependent insulinotropic polypeptide (GIP) (D), and glucagon (E) after the ingestion of 75-g oral glucose in 13 women with impaired glucose tolerance (IGT) (square symbols) and 11 control women (round symbols). Data are presented as means±standard deviation; P values were calculated using repeated measures ANOVA. aSignificant differences at individual time points (P<0.05 by one-way ANOVA).

Table 1
Characteristics of the women with previous gestational diabetes mellitus (pGDM) and of control subjects
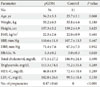
Table 2
Insulin sensitivity/resistance and area under the curve (AUC) of the incretin hormones

Insulin resistance determined by homeostasis model assessment [HOMA-IR=fasting glucose (X mg/dL÷18) (mmol/L)×fasting insulin (µIU/mL)/22.5].
Insulinogenic index=Δinsulin/Δglucose (U/mol).
pGDM, previous gestational diabetes mellitus; HOMA-IR, homeostasis model assessment-insulin resistance; OGTT, oral glucose tolerance test; GLP-1, glucagon like pepide1; GIP, glucose-dependent insulinotropic polypeptide.
ACKNOWLEDGMENT
This study was supported by Reseach Grant (02-2005-003) from the Seoul National University Bundang Hospital, and Grant 00-PJ3-PG6-GN07-001 from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Korea.
References
1. Metzger BE. Summary and recommendations of the Third International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes. 1991. 40:Suppl 2. 197–201.
2. Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005. 115:485–491.
3. Hong ES, Lee HJ, Hong YS, Sung EA, Jang YJ. Pospartum assessment of insulin secretion and sensitivity in women with gestational diabetes mellitus (GDM). J Korean Diabetes Assoc. 2002. 26:319–327.
4. Lim S, Choi SH, Park YJ, Park KS, Lee HK, Jang HC, Cho NH, Metzger BE. Visceral fatness and insulin sensitivity in women with a previous history of gestational diabetes mellitus. Diabetes Care. 2007. 30:348–353.
5. Ebert R, Creutzfeldt W. Gastrointestinal peptides and insulin secretion. Diabetes Metab Rev. 1987. 3:1–26.
6. Unger RH, Eisentraut AM. Entero-insular axis. Arch Intern Med. 1969. 123:261–266.
7. Gerich JE. The genetic basis of type 2 diabetes mellitus: impaired insulin secretion versus impaired insulin sensitivity. Endocr Rev. 1998. 19:491–503.
8. Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986. 29:46–52.
9. Vilsboll T, Krarup T, Madsbad S, Holst JJ. Defective amplification of the late phase insulin response to glucose by GIP in obese type II diabetic patients. Diabetologia. 2002. 45:1111–1119.
10. Cypryk K, Vilsboll T, Nadel I, Smyczynska J, Holst JJ, Lewinski A. Normal secretion of the incretin hormones glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 during gestational diabetes mellitus. Gynecol Endocrinol. 2007. 23:58–62.
11. Forbes S, Moonan M, Robinson S, Anyaoku V, Patterson M, Murphy KG, Ghatei MA, Bloom SR, Johnston DG. Impaired circulating glucagon-like peptide-1 response to oral glucose in women with previous gestational diabetes. Clin Endocrinol (Oxf). 2005. 62:51–55.
12. Jang HC, Yim CH, Han KO, Yoon HK, Han IK, Kim MY, Yang JH, Cho NH. Gestational diabetes mellitus in Korea: prevalence and prediction of glucose intolerance at early postpartum. Diabetes Res Clin Pract. 2003. 61:117–124.
13. Jang HC, Cho YM, Park KS, Kim SY, Lee HK, Kim MY, Yang JH, Shin SM. Pregnancy outcome in Korean women with gestational diabetes mellitus diagnosed by the carpenter-coustan criteria. J Korean Diabetes Assoc. 2004. 28:122–130.
14. Katsuki A, Sumida Y, Gabazza EC, Murashima S, Furuta M, Araki-Sasaki R, Hori Y, Yano Y, Adachi Y. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001. 24:362–365.
15. Jensen SL, Nielsen OV, Kuhl C. The enteral insulin-stimulation after pancreas transplantation in the pig. Diabetologia. 1976. 12:617–620.
16. Meier JJ, Gallwitz B, Askenas M, Vollmer K, Deacon CF, Holst JJ, Schmidt WE, Nauck MA. Secretion of incretin hormones and the insulinotropic effect of gastric inhibitory polypeptide in women with a history of gestational diabetes. Diabetologia. 2005. 48:1872–1881.
17. Nauck MA, El-Ouaghlidi A, Gabrys B, Hucking K, Holst JJ, Deacon CF, Gallwitz B, Schmidt WE, Meier JJ. Secretion of incretin hormones (GIP and GLP-1) and incretin effect after oral glucose in first-degree relatives of patients with type 2 diabetes. Regul Pept. 2004. 122:209–217.
18. Nyholm B, Walker M, Gravholt CH, Shearing PA, Sturis J, Alberti KG, Holst JJ, Schmitz O. Twenty-four-hour insulin secretion rates, circulating concentrations of fuel substrates and gut incretin hormones in healthy offspring of type II (non-insulin-dependent) diabetic parents: evidence of several aberrations. Diabetologia. 1999. 42:1314–1323.
19. Hansotia T, Drucker DJ. GIP and GLP-1 as incretin hormones: lessons from single and double incretin receptor knockout mice. Regul Pept. 2005. 128:125–134.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download