Abstract
Background
Small dense low density lipoprotein (sdLDL) has recently emerged as an important risk factor of coronary heart disease.
Methods
The mean LDL particle size was measured in 203 patients with type 2 diabetes mellitus (T2DM) and 212 matched subjects without diabetes using polyacrylamide tube gel electrophoresis. Major vascular complications were defined as stroke, angiographically-documented coronary artery disease or a myocardial infarction. Peripheral vascular stenosis, carotid artery stenosis (≥50% in diameter) or carotid artery plaque were considered minor vascular complications. Overall vascular complications included both major and minor vascular complications.
Results
Diabetic patients had significantly smaller mean-LDL particle size (26.32 nm vs. 26.49 nm) and a higher percentage of sdLDL to total LDL compared to those of subjects without diabetes (21.39% vs. 6.34%). The independent predictors of sdLDL in this study were serum triglyceride level and body mass index (odds ratio [OR], 1.020 with P<0.001 and OR 1.152 with P<0.027, respectively). However, no significant correlations were found between sdLDL and major vascular complications (P=0.342), minor vascular complications (P=0.573) or overall vascular complications (P=0.262) in diabetic subjects.
Conclusion
Diabetic patients had a smaller mean-LDL particle size and higher proportion of sdLDL compared to those of subjects without diabetes. Obese diabetic patients with hypertriglyceridemia have an increased risk for atherogenic small dense LDL. However, we could not verify an association between LDL particle size and vascular complications in this study.
Coronary heart disease (CHD) is the leading cause of death in patients with type 2 diabetes (T2DM). Individuals with diabetes have a 2- to 4-fold increased risk of CHD compared with that of non-diabetic individuals [1-3]. Moreover, there is evidence that patients with diabetes with no history of CHD had the same risk of myocardial infarction (MI) as that observed in non-diabetic subjects with a history of MI [4]. This similar level of risk of diabetes and previous CHD has led to the suggestion that individuals with diabetes should be treated as CHD-risk equivalents [5]. Several large-scale clinical trials have shown that reduction of low density lipoprotein (LDL) cholesterol level is crucial in reducing CHD morbidity and mortality in both primary and secondary prevention settings [5]. However, using LDL level alone is insufficient as a method of identifying individuals with incident CHD since approximately 50% of these events occur in subjects with normal LDL level [3]. This has led to the hypothesis that other factors may be implicated in the pathogeneses of atherosclerosis and CHD. One of the underlying reasons for this increased risk in T2DM patients is atherogenic dyslipidemia, which is common in T2DM patients and is characterized by a low plasma level of high density lipoprotein (HDL) cholesterol, increased levels of serum triglycerides, specifically very low density lipoprotein triglycerides, and increased levels of small dense LDL (sdLDL) particle [6]. DM itself increases the level of sdLDL but not that of LDL. The sdLDLs found in T2DM are more atherogenic [7,8] and are associated with more than a 3-fold increase in the risk of CHD [9-14]. The physicochemical properties of sdLDL particles provide the potential for increased atherogenicity. sdLDLs have easier access to the subendothelial spaces in the arterial walls and exhibit enhanced binding to intimal proteoglycans [15,16]. sdLDLs also exhibit increased susceptibility to oxidation and uptake by macrophages, therefore facilitating the formation of foam cells [17]. Moreover, sdLDL particles undergo decreased recognition by the LDL receptor, which results in an increased plasma half-life that may enhance their 'anchorage' to the arterial wall, as well as increased oxidation [18,19]. Furthermore, sdLDL exhibits retarded metabolism compared with that of medium-sized LDL [20] and is associated with an elevated fibrinogen level [21]. Finally, an inverse relationship exists between LDL particle size and level of plasminogen activator inhibitor-1, a factor associated with impaired fibrinolysis and atherosclerotic disease [22]. However, data regarding the relationship between LDL particle size and vascular complications in a Korean diabetic population are limited to a relatively small number of patients [23-25]. Therefore, we sought to estimate LDL particle size and risk factors associated with sdLDL in a larger number of Korean type 2 diabetic patients and matched non-diabetic controls.
We performed a study with 203 consecutive T2DM patients (145 males and 58 females) who had visited the Samsung Medical Center in Seoul, Republic of Korea for the treatment of diabetes between the dates of July 2009 and August 2010. Exclusion criteria included the use of lipid-lowering agents (statins, fibrate, nicotinic acid or ezetimibe) that might alter the measurements performed in this study, as well as a medical history of severe renal disease, severe hepatic disease, infectious disease or malignancy. Blood samples were collected from these patients to measure fasting serum glucose, HbA1c, total cholesterol, triglycerides (TG), HDL, LDL, and LDL particle size. We retrospectively evaluated the medical records of each patient for information regarding their medication use, duration of having diabetes, history of vascular complications, body mass index (BMI), urine albumin/creatinine ratio, smoking status (current, ex- or never-smoker) and alcohol consumption (unit per day) in order to identify and evaluate the risk factors for vascular complications. Subjects without diabetes were matched for sex and by age group (e.g., 40 to 49 years, 50 to 59 years) to the 203 diabetic patients to clarify the impact of DM on LDL size. The non-diabetic control subjects (defined as those with no history of DM diagnosis or medication and an HbA1c level <5.7%) were identified from the database of the Center for Health Promotion at the Samsung Medical Center. The aforementioned exclusion criteria were also applied to the non-diabetic subjects. Based on age and sex, 609 individuals were eligible for matching, and 212 of these were further selected using a statistical matching tool. After routine analysis was performed on these patients, the remaining blood samples were collected for LDL subfraction analysis. Major vascular complications were defined as stroke, angiographically-documented coronary artery disease or myocardial infarction. Minor vascular complications were defined as peripheral vascular stenosis, carotid artery stenosis (≥50% in diameter) or carotid artery plaque. Data on vascular complications were collected from the patient's medical records. Overall vascular complications included both major and minor vascular complications. The Institutional Review Board at Samsung Medical Center approved the study protocol (2010-08-152-001).
The LDL subfraction was analyzed using polyacrylamide tube gel electrophoresis (Quantimetrix Lipoprint™; LDL System, Redondo Beach, CA, USA) [6], a method that has been reported to be simple, cost-effective and free of inter-individual interpretation bias [26]. The samples were then categorized as either phenotype A or B based on mean LDL particle size. LDL subtypes 1 to 2 were predominantly large, buoyant LDLs; subtypes 3 to 7 were predominantly sdLDLs. The mean LDL particle size for 'phenotype A' was greater than 26.5 nm (265 Å), hence considered 'large, buoyant LDL dominant,' while the mean value of particle size for 'phenotype B' was less than 26.5 nm, and was therefore considered, 'small, dense LDL dominant.' The sdLDL (subtypes 3 to 7) percentage of total LDL was measured as follows:
Statistical analyses were performed using the PASW Statistics 18.0 program for Windows (SPSS Inc., Chicago, IL, USA). We conducted a one-sample Kolmogorov-Smirnov test to verify the distribution of the data. All data were summarized as the mean±standard deviation or as a percentage, while those with a skewed distribution were described as a median (IQR). The chi-square test was used to compare the differences in variables between the two groups. Student's t-test was used for continuous, normal variables. The Mann-Whitney test was used to test independent relationships between the variables that did not demonstrate normality. A two-sided P value less than 0.05 was considered statistically significant. Variables were entered into a multiple logistic regression analysis if their P value was less than 0.05 in the univariate analysis in order to assess independent associations between risk factors, size of LDL particles and vascular complications. Spearman's correlation analyses were used to assess the correlations between various parameters and LDL particle size.
The clinical and metabolic characteristics of the 203 diabetic patients and the 212 matched control subjects without diabetes are shown in Table 1. Patients with diabetes had significantly smaller mean LDL particle size (26.32 nm vs. 26.49 nm) and a higher percentage of sdLDL to total LDL compared to those of the non-diabetic controls (21.39% vs. 6.34%). T2DM patients had higher BMIs and levels of fasting glucose, HbA1c and TG than did the non-diabetic controls. Total cholesterol, HDL, and LDL levels were higher in those without diabetes, who also had the higher percentage of current smokers. Sex, age, proportion of patients with sdLDL (phenotype B) and alcohol consumption did not differ significantly between the two groups. Comparison of the baseline characteristics between the A and B LDL phenotypes in diabetic patients are summarized in Table 2. The phenotype B group had a higher BMI and higher TG and LDL levels than did the phenotype A group. Sex, age, duration of diabetes, fasting glucose level, HbA1c, total cholesterol, LDL, urine albumin/creatinine ratio, smoking status and alcohol consumption did not differ between the two groups. Multivariate logistic regression analysis in the diabetic group identified TG (odds ratio [OR], 1.020; 95% confidence interval [CI], 1.012 to 1.027; P<0.001) and BMI (OR, 1.152; 95% CI, 1.016 to 1.305; P=0.027) as independent risk factors for sdLDL. The OR was calculated according to TG level and BMI as a practical method of evaluation (Table 3). Diabetic patients with a serum TG level ≥200 mg/dL or a BMI ≥30 kg/m2 had a 17-fold and a 4-fold greater risk for sdLDL, respectively. However, no significant association was found between sdLDL in diabetic subjects and major vascular complications (P=0.342), minor vascular complications (P=0.573) or overall vascular complications (P=0.262, Table 4). Multiple stepwise regression analyses of the major vascular complications and other risk factors were also performed. Age was the only independent predictive factor of major vascular complications in diabetic patients (OR, 1.127; 95% CI, 1.055 to 1.205; P<0.001). Fasting serum glucose level and levels of HbA1c and lipids were not significantly associated with vascular complications (data not shown). The variables significantly correlated with LDL size in the Spearman's correlation analysis are presented in Table 5. Among these variables, TG level had the strongest correlation with mean LDL particle size in diabetic patients (correlation coefficient=-0.558, P<0.001), as depicted in Fig. 1.
The results from the present study show that diabetic patients have smaller LDL particles and a higher level of sdLDL than do non-diabetic subjects. This study also confirmed an association between plasma TG concentration and LDL particle size. However, no significant correlation was found between sdLDL and vascular complications in diabetic patients.
The prevalence of sdLDL was 53.2% and 49.1% in Korean type 2 diabetic patients and non-diabetic subjects, respectively. This figure is higher than those observed in previous studies [27,28] and is much higher than that in a report of healthy, non-diabetic Korean adults [29]. An explanation for the difference between the reported studies and our own may be due to the differences in the populations studied. Ethnic, dietary and genetic factors play an important role in the phenotypic expression of the plasma lipid profile. However, the well-known high prevalence of sdLDL in patients with diabetes was observed in this study, as can be seen by the smaller mean LDL particle size and higher percentage of sdLDL in these patients. As expected, T2DM patients had lower HDL level, higher BMI and much higher serum glucose and HbA1c levels. However, total cholesterol and LDL levels were higher in the non-diabetic subjects. This may be explained by the higher TG levels and the effects of multiple medications in diabetic patients. Although subjects were excluded from the study if they were taking lipid-lowering drugs, we may have overlooked the effects of hypoglycemic agents (such as thiazolidinediones) on the patients' lipid profiles. Still, it is noteworthy that those with diabetes had smaller LDL particles and a higher proportion of sdLDL even though their total cholesterol and LDL levels were lower than those in the control group. Diabetic patients with sdLDL had higher TG levels and BMI and lower HDL compared to those with large buoyant LDL, a finding that is consistent with those of other studies [30,31]. Those with metabolic syndrome are well known for having an increased risk for CHD and reduced LDL size [32,33]. The low percentage of current smoker among our diabetic patients may have resulted from our consistent advice to stop smoking.
The independent predictors of sdLDL in T2DM patients were serum TG level and BMI (OR, 1.020, with P<0.001; and OR, 1.152, with P=0.027, respectively), which is consistent with the findings of previous studies on conditions associated with sdLDL [20,30,31,34-36]. Metabolic syndrome, insulin-resistance and decreased adiponectin are plausible explanations for this result. In regard to clinical guidance, we analyzed the ORs for TG and BMI according to level and found that diabetic patients with TG level ≥150 mg/dL and BMI ≥30 kg/m2 were more likely to have sdLDL. Thus, we should educate patients to lose weight and improve dyslipidemia through exercise and dietary restriction with the aim of blood glucose control. Collaboration with the Department of Sports Medicine and Nutrition is essential for achieving this goal.
Contrary to our expectations, LDL particle size and small LDL particle fraction failed to show any meaningful correlation with vascular complications. This may be due to the cross-sectional design of our study and because of the recently improved glycemic control guidelines, which may have reduced vascular complications with the wide prescription of medication such as aspirin. Our attempt to divide the diabetic patients into three or four groups according to mean LDL particle size in order to analyze the correlations with vascular complications did not show any significant results (data not shown). A recent article by Ip et al. [37] has found that, while the LDL particle number was associated with incident cardiovascular disease, the LDL particle size and the small LDL particle fraction were not consistently associated with disease incidence. Furthermore, there have been no studies to date that have performed an adequate analysis to determine the relative or incremental value of LDL subfraction measurement as a predictor of cardiovascular disease compared with traditional risk factors. These studies also do not emphasize the clinical value of providing treatment based on results of LDL subfraction testing. From the current results, we cannot conclude that sdLDL is not an independent predictor of vascular complications. Longitudinal studies with a larger number of patients are essential to determine whether sdLDL play a crucial role in the development of CHD. However, it would be unethical to design a study in which known risk factors are not treated in order to verify the clinical value of LDL particle size. Therefore, we are planning to closely observe these patients until death and to then re-evaluate the significance of the sdLDL.
In agreement with other studies [38,39], TG concentration was the strongest determinant of LDL particle size in the present study. This result may be due to the association of hypertriglyceridemia with sdLDL since the metabolism of TG-rich lipoprotein is the major determinant of sdLDL particle formation. LDL metabolism and particle size are associated with very low density lipoprotein (VLDL) metabolism. The precursor of sdLDL, β-pool LDL, is synthesized from VLDL1, and the precursor of the large buoyant LDL, α-pool LDL is synthesized from VLDL2. The synthesis of VLDL1 is increased at higher TG concentrations; thus, the synthesis of β-pool LDL is also increased when TG concentration is elevated. This β-pool LDL donates cholesterol to VLDL1 through a reaction with cholesteryl ester transfer protein and receives TG from VLDL1. Consequently, the β-pool LDL is converted to LDL when there is a high TG concentration and a low level of cholesterol. The TG is hydrolyzed by hepatic lipase and is then converted to small diameter sdLDL [40].
We acknowledge the limitation of using a cross-sectional study design since we were not able to fully demonstrate the association between vascular complications and LDL particle size. A larger prospective study is needed to fully validate this association.
In conclusion, diabetic patients had smaller mean-LDL particle size and higher proportion of sdLDL compared to those of non-diabetic controls. Obese diabetic patients with hypertriglyceridemia have an increased risk for atherogenic sdLDL. However, we could not verify an association between LDL particle size and vascular complications in this study.
Figures and Tables
Fig. 1
Correlation analysis between triglycerides (TG) level and low density lipoprotein (LDL) size in the diabetic group.

Table 2
Comparison of the clinical and metabolic characteristics between phenotypes A and B in diabetic patients
Table 3
Multivariate logistic regression analysis of small dense LDL (phenotype B) in diabetic patients (n=203)

ACKNOWLEDGMENTS
This study was supported in part by a Clinical Research Development Project grant (CRS110-22-1) of Samsung Medical Center.
References
1. Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993. 16:434–444.
2. American Diabetes Association. Consensus development conference on the diagnosis of coronary heart disease in people with diabetes: 10-11 February 1998, Miami, Florida. Diabetes Care. 1998. 21:1551–1559.
3. Kannel WB. Range of serum cholesterol values in the population developing coronary artery disease. Am J Cardiol. 1995. 76:69C–77C.
4. Haffner SM, D'Agostino R Jr, Goff D, Howard B, Festa A, Saad MF, Mykkanen L. LDL size in African Americans, Hispanics, and non-Hispanic whites: the insulin resistance atherosclerosis study. Arterioscler Thromb Vasc Biol. 1999. 19:2234–2240.
5. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001. 285:2486–2497.
6. Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Despres JP. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Québec Cardiovascular Study. Circulation. 1997. 95:69–75.
7. Krauss RM. Dense low density lipoproteins and coronary artery disease. Am J Cardiol. 1995. 75:53B–57B.
8. Krauss RM. Dietary and genetic effects on low-density lipoprotein heterogeneity. Annu Rev Nutr. 2001. 21:283–295.
9. Lamarche B, Tchernof A, Mauriege P, Cantin B, Dagenais GR, Lupien PJ, Despres JP. Fasting insulin and apolipoprotein B levels and low-density lipoprotein particle size as risk factors for ischemic heart disease. JAMA. 1998. 279:1955–1961.
10. Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996. 276:882–888.
11. Austin MA, Hokanson JE, Brunzell JD. Characterization of low-density lipoprotein subclasses: methodologic approaches and clinical relevance. Curr Opin Lipidol. 1994. 5:395–403.
12. Lamarche B, Lemieux I, Despres JP. The small, dense LDL phenotype and the risk of coronary heart disease: epidemiology, patho-physiology and therapeutic aspects. Diabetes Metab. 1999. 25:199–211.
13. Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996. 276:875–881.
14. Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. Am J Cardiol. 2002. 90:89–94.
15. Nielsen LB. Transfer of low density lipoprotein into the arterial wall and risk of atherosclerosis. Atherosclerosis. 1996. 123:1–15.
16. Anber V, Millar JS, McConnell M, Shepherd J, Packard CJ. Interaction of very-low-density, intermediate-density, and low-density lipoproteins with human arterial wall proteoglycans. Arterioscler Thromb Vasc Biol. 1997. 17:2507–2514.
17. Krauss RM. Atherogenic lipoprotein phenotype and diet-gene interactions. J Nutr. 2001. 131:340S–343S.
18. Galeano NF, Al-Haideri M, Keyserman F, Rumsey SC, Deckelbaum RJ. Small dense low density lipoprotein has increased affinity for LDL receptor-independent cell surface binding sites: a potential mechanism for increased atherogenicity. J Lipid Res. 1998. 39:1263–1273.
19. Toyota Y, Yamamura T, Miyake Y, Yamamoto A. Low density lipoprotein (LDL) binding affinity for the LDL receptor in hyperlipoproteinemia. Atherosclerosis. 1999. 147:77–86.
20. Rizzo M, Berneis K. Should we measure routinely the LDL peak particle size. Int J Cardiol. 2006. 107:166–170.
21. Maki KC, Davidson MH, Marx P, Cyrowski MS, Maki A. Association between elevated plasma fibrinogen and the small, dense low-density lipoprotein phenotype among postmenopausal women. Am J Cardiol. 2000. 85:451–456.
22. Festa A, D'Agostino R Jr, Mykkanen L, Tracy R, Howard BV, Haffner SM. Low-density lipoprotein particle size is inversely related to plasminogen activator inhibitor-1 levels. The Insulin Resistance Atherosclerosis Study. Arterioscler Thromb Vasc Biol. 1999. 19:605–610.
23. Lee W, Min WK, Chun S, Jang S, Kim JQ, Lee DH, Park JY, Park H, Son JE. Low-density lipoprotein subclass and its correlating factors in diabetics. Clin Biochem. 2003. 36:657–661.
24. Yoon Y, Song J, Park HD, Park KU, Kim JQ. Significance of small dense low-density lipoproteins as coronary risk factor in diabetic and non-diabetic Korean populations. Clin Chem Lab Med. 2005. 43:431–437.
25. Kwon SW, Yoon SJ, Kang TS, Kwon HM, Kim JH, Rhee J, Lee SJ, Park JK, Lim JY, Yoon YW, Hong BK. Significance of small dense low-density lipoprotein as a risk factor for coronary artery disease and acute coronary syndrome. Yonsei Med J. 2006. 47:405–414.
26. Hoefner DM, Hodel SD, O'Brien JF, Branum EL, Sun D, Meissner I, McConnell JP. Development of a rapid, quantitative method for LDL subfractionation with use of the Quantimetrix Lipoprint LDL System. Clin Chem. 2001. 47:266–274.
27. Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990. 82:495–506.
28. Campos H, Blijlevens E, McNamara JR, Ordovas JM, Posner BM, Wilson PW, Castelli WP, Schaefer EJ. LDL particle size distribution. Results from the Framingham Offspring Study. Arterioscler Thromb. 1992. 12:1410–1419.
29. Park JS, Park J, Kim CS, Cho MH, Kim HJ, Kim JH, Ahn CW, Kim KR, Cha BS, Lim SK, Lee HC. Relationship of low-density lipoprotein particle size to insulin resistance and intima-media thickness in nondiabetic Koreans. Metabolism. 2006. 55:1610–1615.
30. Miller WM, Nori-Janosz KE, Lillystone M, Yanez J, McCullough PA. Obesity and lipids. Curr Cardiol Rep. 2005. 7:465–470.
31. Kang HS, Gutin B, Barbeau P, Litaker MS, Allison J, Le NA. Low-density lipoprotein particle size, central obesity, cardiovascular fitness, and insulin resistance syndrome markers in obese youths. Int J Obes Relat Metab Disord. 2002. 26:1030–1035.
32. Kolovou GD, Anagnostopoulou KK, Cokkinos DV. Pathophysiology of dyslipidaemia in the metabolic syndrome. Postgrad Med J. 2005. 81:358–366.
33. Hulthe J, Bokemark L, Wikstrand J, Fagerberg B. The metabolic syndrome, LDL particle size, and atherosclerosis: the Atherosclerosis and Insulin Resistance (AIR) study. Arterioscler Thromb Vasc Biol. 2000. 20:2140–2147.
34. Li J, Kondo A, Maekawa M, Kanamori M, Kanno T. Hypertriglyceridemia characterized by low-density lipoprotein phenotype and lipoprotein lipase gene mutation. Clin Chem Lab Med. 2000. 38:1263–1270.
35. Krauss RM. Dietary and genetic probes of atherogenic dyslipidemia. Arterioscler Thromb Vasc Biol. 2005. 25:2265–2272.
36. Gazi IF, Milionis HJ, Filippatos TD, Tsimihodimos V, Kostapanos MS, Doumas M, Tselepis AD, Elisaf M. Hypertriglyceridaemic waist phenotype criteria and prevalent metabolic triad in women. Diabetes Metab Res Rev. 2008. 24:223–230.
37. Ip S, Lichtenstein AH, Chung M, Lau J, Balk EM. Systematic review: association of low-density lipoprotein subfractions with cardiovascular outcomes. Ann Intern Med. 2009. 150:474–484.
38. Lahdenpera S, Sane T, Vuorinen-Markkola H, Knudsen P, Taskinen MR. LDL particle size in mildly hypertriglyceridemic subjects: no relation to insulin resistance or diabetes. Atherosclerosis. 1995. 113:227–236.
39. Suehiro T, Ohguro T, Sumiyoshi R, Yasuoka N, Nakauchi Y, Kumon Y, Hashimoto K. Relationship of low-density lipoprotein particle size to plasma lipoproteins, obesity, and insulin resistance in Japanese men. Diabetes Care. 1995. 18:333–338.
40. Packard CJ, Demant T, Stewart JP, Bedford D, Caslake MJ, Schwertfeger G, Bedynek A, Shepherd J, Seidel D. Apolipoprotein B metabolism and the distribution of VLDL and LDL subfractions. J Lipid Res. 2000. 41:305–318.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


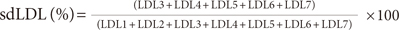



 XML Download
XML Download