Abstract
Cardiovascular disease (CVD), a leading cause of death in patients with diabetes mellitus, has several pathogenic mechanisms that are well established. However, the traditional hypoglycemic agents do not have proven positive effects on macrovascular disease. Novel therapeutic agents target the incretin pathway including the glucagon-like peptide 1 (GLP-1) receptor (GLP-1R) agonists and the dipeptidyl peptidase-4 inhibitors. The glucose-regulatory actions of these agents function by increasing insulin secretion and suppressing glucagon. They also act to increase weight loss not only by inhibiting gastric emptying, but also by reducing appetite. Although GLP-1 and GLP-1R agonists have demonstrated beneficial effects on myocardium and vascular endothelium including coronary and peripheral mouse vessels, they also have anti-inflammatory and anti-atherogenic actions. These agents also have positive effects on the lipid profile and blood pressure. Although these cardioprotective actions seem to be beyond the effects of glucose control and weight loss, they are mediated through GLP-1R- or GLP-1R-independent actions of cleaved GLP-1 (9-36). Larger randomized controlled trials are necessary to elucidate the clinical promise of these beneficial CVD effects.
Patients with diabetes mellitus (DM) have a significantly increased risk of morbidity and mortality associated with not only cardiovascular disease (CVD), but also stroke, causing 75% of all deaths [1]. These patients also have a 3 to 5-fold increased incidence of death from coronary heart disease compared with nondiabetic subjects [2,3]. The pathogenic mechanisms for developing atherosclerosis in DM include endothelial dysfunction, oxidative stress, impaired vasodilation, and the increased generation of glycosylated products. Diabetes often coexists with other well-established risk factors for CVD including hypertension and dyslipidemia. The management of hypertension and elevated blood lipid levels is effective in improving cardiovascular outcome. However, little is known regarding the long-term and beneficial effects of traditional anti-diabetic agents on macrovascular disease [4]. In some instances, the chronic use of hypoglycemic agents may induce negative cardiovascular outcomes, despite improvement in hyperglycemia [5].
Recently, a novel class of incretin-based hypoglycemic agents has been developed. Incretins, gut-derived hormones, predominantly glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide, are released in response to ingested nutrients, mainly glucose. Incretins not only stimulate postprandial insulin release from pancreatic β-cells [6] and inhibit glucagon secretion [7,8], but also inhibit gastric emptying [9]. Active isoforms of GLP-1 include GLP-1 (7-36) amide and GLP-1 (7-37). Approximately 80% of the circulating active GLP-1 is GLP-1 (7-36) amide, which is rapidly cleaved by dipeptidyl peptidase-4 (DPP-4) to GLP-1 (9-36), which does not interact with the known GLP-1 receptor [6,10,11]. The GLP-1 stimulates the glucagon-like peptide 1 receptor (GLP-1R), a G protein-coupled receptor that is expressed in islet β-cells. Other areas for the GLP-1R include not only the central (hypothalamus) and peripheral nervous systems, but also the gastrointestinal tract, lung, heart and vasculature [6,12,13]. GLP-1R activation stimulates adenylate cyclase, which increases cAMP. The increased cAMP activates protein kinase A, which then sensitizes the beta cells to glucose-stimulated insulin secretion. GLP-1 administration not only produces weight loss associated with reduced food intake [14,15], but also promotes insulin biosynthesis and β-cell proliferation [16,17]. It has also been suggested that GLP-1 may have neuroprotective, cardioprotective, and vasodilatory benefits [18]. Apart from the indirect effect of GLP-1 on CVD outcomes through improved hyperglycemia, there is accumulating evidence from both experimental and clinical studies suggesting a direct influence on the myocardium as well [19,20].
The present overview considers the pleiotropic actions of GLP-1 and GLP-1R agonists on the cardiovascular system including risk factors and outcomes.
Since the associations of hyperglycemia and dysmetabolism with cardiovascular risk have been demonstrated in a number of trials [21-23], it would be useful to evaluate whether GLP-1 and GLP-1R agonists reduce cardiovascular risk.
Plasminogen activator inhibitor type-1 (PAI-1) levels have been implicated in endothelial cell dysfunction. Liu et al. [24] reported that treatment with GLP-1 in human umbilical vein endothelial cells (HUVEC) attenuated the ability of tumor necrosis factor-alpha (TNF-α) to induce the PAI-1 gene and its protein expression. The above treatment also inhibited Akt phosphorylation. In addition, liraglutide, a long-acting GLP-1 analogue, not only inhibited TNF-α or the hyperglycemia-mediated induction of PAI-1, the intercellular adhesion molecule-1 and the vascular cell adhesion molecule-1 mRNA, but also the protein expression in a human vascular endothelial cell [25].
Arakawa et al. [26] reported that the GLP-1R was abundantly expressed in monocytes/macrophages. This finding could suggest that GLP-1 can directly act on monocytes or macrophages. Continuous infusion of exendin-4, a GLP-1R agonist, for 28 days significantly inhibited monocytic adhesion in the thoracic aortas of C57BL/6 mice without any change in body weight or glucose tolerance. In apoE-/- mice, exendin-4 not only inhibited the monocyte adhesion to the endothelium with downregulation of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), it also reduced atherosclerotic lesions (Fig. 1). The in vitro treatment with exendin-4 suppressed the lipopolysaccharide (LPS)-induced expression of TNF-α and monocyte chemoattractant protein-1 (MCP-1) as well as suppressing the LPS-induced nuclear translocation of proinflammatory transcriptional nuclear factor-κB (NF-κB) p65 in mouse macrophages. This effect was completely abolished by MDL-12330A, a cAMP inhibitor, and PKI14-22, a protein kinase A-specific inhibitor. These results suggest that GLP-1R activation significantly reduces monocyte/macrophage accumulation in the vascular wall. This activation eventually suppressed atherosclerogenesis by inhibiting the inflammatory response in the macrophages via the cAMP/PKA pathway.
In a small randomized study, GLP-1 infusion in 12 patients with type 2 DM (T2DM) and stable coronary artery disease caused a significant increase in the flow-mediated vasodilation (FMD), with no effects on the insulin sensitivity index (ISI). These effects were not seen in healthy subjects. These observations suggest that GLP-1 might potentially improve endothelial dysfunction associated with atherosclerosis but not insulin resistance in patients with T2DM [27]. In a recent randomized study, 128 T2DM patients who received 12 months of either exenatide or glibenclamide showed a significant reduction in body weight (-6.3 kg) and a reduction in hs-CRP (-0.4 mg/L), a marker of inflammation [28].
Meier et al. [29] reported that the GLP-1 administration not only abolished the postprandial increase in triglyceride level (-0.023±0.045 mmol/L, P<0.05), but also suppressed plasma fasting and postprandial non-esterified fatty acid (NEFA) concentrations by 39% and 31%, respectively (P<0.01), in 14 healthy male subjects. After 82 weeks of adjunctive exenatide treatment in 314 obese diabetics treated with sulfonylurea and/or metformin, there was not only a progressive reduction of body weight (-4.4±0.3 kg), a reduction in triglyceride concentration (16%), and a reduction in sitting diastolic blood pressure (-2.7 mm Hg), but also a significant increase in high density lipoprotein cholesterol (12%) [30]. These observations suggest that, even though GLP-1 might improve postprandial lipidemia by delaying gastric emptying, it may also affect the insulin-mediated inhibition of lipolysis. There may also be an increase in triglyceride clearance or a reduction in the endogenous synthesis of triglycerides [29,31,32]. It is suggested that the blood pressure-lowering effect in patients with DM could be caused by GLP-1-induced natriuresis [33] and vasodilatory effects [18] rather than improved insulin resistance [34]. However, the effect of GLP-1 and/or GLP-1R agonists on blood pressure remains to be determined because conflicting data are still being reported [35-38].
Since the GLP-1Rs are also expressed in cardiac tissues, it is feasible that GLP-1 and GLP-1R agonists may mediate cardiac functions. In both the isolated perfused rat heart and the whole animal models of ischemia/reperfusion (I/R) [39], treatment with GLP-1 before ischemia significantly reduced infarct size. The GLP-1R antagonist exendin (9-39) and inhibitors of cAMP (Rp-cAMP), phosphoinositide 3-kinases (LY294002), and p42/44 mitogen-activated protein kinase (UO126) abolished this effect in both heart models. These data suggest that the cardioprotective effects of GLP-1 involve multiple pro-survival kinases rather than noncardiac effects. Timmers et al. [40] examined the efficacy of GLP-1R agonists at reducing infarct size in pigs. The administration of exenatide subsequent to the I/R reduced infarct size by 40% and improved systolic wall thickening, ventricular volumes and myocardial stiffness compared with those in the saline controls. These beneficial myocardial effects of the GLP-1R agonist were associated with the modulation of molecular mechanisms regulating apoptosis. Some of these mechanisms include higher levels of phosphorylated Akt and bcl-2 expression with lower levels of caspase-3 expression and apoptosis-inducing caspases. Additionally, with modulation of oxidative stress, there are higher levels of antioxidant enzymes and reduction of oxidative stress markers. These data suggest these beneficial effects of exenatide on the myocardium could be associated with reduced apoptosis and oxidative stress (Fig. 2).
There are a number of studies with human subjects that evaluated the cardioprotective effects of GLP-1 and GLP-1R agonists. Nikolaidis et al. [41] investigated the 72-hour infusion of GLP-1 (1.5 pmol/kg/min) in 10 patients with not only an acute myocardial infarction, but also a left ventricular ejection fraction (LVEF) <40%. After a successful primary angioplasty significantly improved LVEF (11%) and wall motion score indexes (21%) compared with those of untreated controls, these improvements were maintained for months in some subjects after cessation of GLP-1 treatment. A 5-week infusion of GLP-1 (2.5 pmol/kg/min) in 12 patients with New York Heart Association (NYHA) class III/IV heart failure significantly improved LVEF, maximum myocardial ventilation oxygen consumption (VO2 max), 6-minute walk distance and Minnesota Living with Heart Failure Quality of Life score (MNQOL) in both diabetic and non-diabetic patients. These results indicate that GLP-1 administration might improve left ventricular function, functional status, and quality of life in patients with severe heart failure [42].
Ban et al. [18] localized GLP-1R expression to cardiomyocytes, endothelium and vascular SMCs of the normal adult mouse heart and showed that GLP-1 administration not only increased glucose uptake and cAMP and cGMP release, but also the left ventricular pressure and coronary flow in isolated mouse hearts. The effect of GLP-1R also increased the functional recovery and the cardiomyocyte viability after I/R injury. However, because the increased cAMP might have a long-term harmful effect in the heart, one must investigate the cardiovascular effects of GLP-1. Ban et al. next turned their attention to the role of GLP-1 (9-36), the DPP-4-generated metabolite of GLP-1, to determine if it has an intermediary potential action against I/R injury. The administration of GLP-1 (9-36) after I/R injury increased vasodilatation and coronary flow in wild-type and Glp1r-/- mice. The administration of NO-synthase inhibitor significantly blocked the vasodilatory effects of GLP-1 and GLP-1 (9-36). As shown in Fig. 3, GLP-1 had important cardiovascular actions through two pathways. One of these pathways included a dependence on the GLP-1R for inotropic action, glucose uptake, ischemic preconditioning, and mild vasodilatory actions. The second depended on GLP-1 (9-36) having GLP-1R-independent effects on the post-ischemic recovery of cardiac function and vasodilation acting, at least in part, through a NOS-dependent cGMP formation. A much larger dose of the GLP-1R agonist exendin-4 (5 nmol/L), a potent DPP-4-resistant GLP-1R agonist, was needed to reproduce the protective effect of native GLP-1. These data further suggest that the cardioprotective effects of GLP-1 are mediated, at least in part, by a GLP-1R-independent effect of GLP-1 (9-36) [43].
Current evidence on the effects of GLP-1 on various cardiovascular risk factors is summarized in Table 1 [44]. Incretin, especially the GLP-1-based compounds, appears to have cardioprotective potential independent of weight loss and glycemic control. These cardioprotective effects might be mediated by the expression of the GLP-1R on a number of cardiac and vascular tissues and even by GLP-1R-independent signaling pathways. However, it remains uncertain if the cardiovascular actions of GLP-1 are mediated only by the GLP-1R because GLP-1 (9-36), the metabolite of GLP-1, shares many of the beneficial effects of GLP-1 in animal models but not in humans. While the clinical use of GLP-1R agonists and DPP-4 inhibitors is increasing for the treatment of T2DM, these cardioprotective effects are not the same as those observed with GLP-1. The human studies of incretin-based therapy detecting a cardiovascular endpoint have been very limited so far. Larger, longer-term clinical studies are needed.
Figures and Tables
Fig. 1
Exendin-4 reduced monocyte adhesion to the endothelium and atherosclerotic lesions in apoE-/- mice after 28-day treatment. (A) En face immunohistochemical staining of Mac-2 antibody of the aorta (n=7). (B) mRNA expression levels of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) (n=5-7). (C) Aortic sinuses stained with oil red O and the mean area of oil red O-positive lesions (n=20). Data are mean±SEM. aP<0.05 vs. control (Adapted from Arakawa M, et al. Diabetes 2010;59:1030-7) [26].
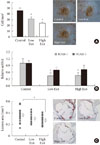
Fig. 2
Exenatide treatment after 75 minutes of coronary artery ligation and subsequent reperfusion in pigs. Myocardial infarct size quantification as a percentage of the area at risk (AAR) (A) and as a percentage of the total left ventricle (LV) (B). PBS (n=9); exenatide (n=9). Representative pictures after Evans blue and triphenyltetrazolium chloride (C, D). Blue indicates non-threatened myocardium, red indicates the noninfarcted area within the area at risk, and white indicates myocardial infarction. Quantifications of immunostaining for 8-hydroxydeoxy-guanosine (E) and terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay (F) (Adapted from Gill A, et al. Cardiovasc Diabetol 2010;9:6) [38].
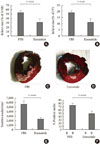
Fig. 3
Physiological cardioprotective actions (A) and proposed mechanisms of glucagon-like peptide 1 (GLP-1) through a novel two-pathway (B). GLP-1R, GLP-1 receptor; DPP-4, dipeptidyl peptidase-4, BP, blood pressure (Modified from Mudaliar S, et al. Am J Med 2009;122(6 Suppl):S25-36, and Ban K, et al. J Am Soc Hypertens 2009;3:245-59. Used with permission) [31,43].

Table 1
Current evidence on the actions of glucagon-like peptide 1 (GLP-1) and GLP-1 receptor (GLP-1R) agonist relevant for cardiovascular risk (Modified from Mannucci E, et al. Nutr Metab Cardiovasc Dis 2008;18:639-45, with permission from Elsevier) [44]

References
1. Grobbee DE. How to ADVANCE prevention of cardiovascular complications in type 2 diabetes. Metabolism. 2003. 52:8 Suppl 1. 24–28.
2. Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993. 16:434–444.
3. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998. 339:229–234.
4. Fisman EZ, Motro M, Tenenbaum A. Non-insulin antidiabetic therapy in cardiac patients: current problems and future prospects. Adv Cardiol. 2008. 45:154–170.
5. Fisman EZ, Tenenbaum A, Motro M, Adler Y. Oral antidiabetic therapy in patients with heart disease: a cardiologic standpoint. Herz. 2004. 29:290–298.
6. Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999. 20:876–913.
7. Matsuyama T, Komatsu R, Namba M, Watanabe N, Itoh H, Tarui S. Glucagon-like peptide-1 (7-36 amide): a potent glucagonostatic and insulinotropic hormone. Diabetes Res Clin Pract. 1988. 5:281–284.
8. Weir GC, Mojsov S, Hendrick GK, Habener JF. Glucagon-like peptide I (7-37) actions on endocrine pancreas. Diabetes. 1989. 38:338–342.
9. Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci. 1993. 38:665–673.
10. Wideman RD, Kieffer TJ. Mining incretin hormone pathways for novel therapies. Trends Endocrinol Metab. 2009. 20:280–286.
11. Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagonlike peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995. 80:952–957.
12. Tomas E, Habener JF. Insulin-like actions of glucagon-like peptide-1: a dual receptor hypothesis. Trends Endocrinol Metab. 2010. 21:59–67.
13. Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005. 1044:127–131.
14. Davis HR Jr, Mullins DE, Pines JM, Hoos LM, France CF, Compton DS, Graziano MP, Sybertz EJ, Strader CD, Van Heek M. Effect of chronic central administration of glucagon-like peptide-1 (7-36) amide on food consumption and body weight in normal and obese rats. Obes Res. 1998. 6:147–156.
15. Larsen PJ, Fledelius C, Knudsen LB, Tang-Christensen M. Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes. 2001. 50:2530–2539.
16. Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003. 278:471–478.
17. Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999. 48:2270–2276.
18. Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008. 117:2340–2350.
19. Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996. 137:2968–2978.
20. Wei Y, Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995. 358:219–224.
21. Patsch JR, Miesenbock G, Hopferwieser T, Muhlberger V, Knapp E, Dunn JK, Gotto AM Jr, Patsch W. Relation of triglyceride metabolism and coronary artery disease: studies in the postprandial state. Arterioscler Thromb. 1992. 12:1336–1345.
22. Karpe F, Steiner G, Uffelman K, Olivecrona T, Hamsten A. Postprandial lipoproteins and progression of coronary atherosclerosis. Atherosclerosis. 1994. 106:83–97.
23. Bell DS, O'Keefe JH, Jellinger P. Postprandial dysmetabolism: the missing link between diabetes and cardiovascular events? Endocr Pract. 2008. 14:112–124.
24. Liu H, Hu Y, Simpson RW, Dear AE. Glucagon-like peptide-1 attenuates tumour necrosis factor-alpha-mediated induction of plasminogen activator inhibitor-1 expression. J Endocrinol. 2008. 196:57–65.
25. Liu H, Dear AE, Knudsen LB, Simpson RW. A long-acting glucagon-like peptide-1 analogue attenuates induction of plasminogen activator inhibitor type-1 and vascular adhesion molecules. J Endocrinol. 2009. 201:59–66.
26. Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R, Watada H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010. 59:1030–1037.
27. Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, Sjoholm A. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004. 287:E1209–E1215.
28. Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Ragonesi PD, Querci F, Franzetti IG, Gadaleta G, Ciccarelli L, Piccinni MN, D'Angelo A, Cicero AF. Exenatide versus glibenclamide in patients with diabetes. Diabetes Technol Ther. 2010. 12:233–240.
29. Meier JJ, Gethmann A, Gotze O, Gallwitz B, Holst JJ, Schmidt WE, Nauck MA. Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia. 2006. 49:452–458.
30. Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006. 8:436–447.
31. Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, Schmiegel WH. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997. 273(5 Pt 1):E981–E988.
32. Mudaliar S, Henry RR. Incretin therapies: effects beyond glycemic control. Am J Med. 2009. 122:6 Suppl. S25–S36.
33. Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B, Beglinger C. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab. 2004. 89:3055–3061.
34. Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, Roman RJ. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003. 21:1125–1135.
35. Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002. 110:43–52.
36. Barragan JM, Rodriguez RE, Blazquez E. Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7-36) amide in rats. Am J Physiol. 1994. 266(3 Pt 1):E459–E466.
37. Okerson T, Yan P, Stonehouse A, Brodows R. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am J Hypertens. 2010. 23:334–339.
38. Gill A, Hoogwerf BJ, Burger J, Bruce S, Macconell L, Yan P, Braun D, Giaconia J, Malone J. Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus: a double-blind, placebo-controlled, randomized pilot study. Cardiovasc Diabetol. 2010. 9:6.
39. Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005. 54:146–151.
40. Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek JJ, Doevendans PA, Pasterkamp G, Hoefer IE. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009. 53:501–510.
41. Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004. 109:962–965.
42. Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006. 12:694–699.
43. Ban K, Hui S, Drucker DJ, Husain M. Cardiovascular consequences of drugs used for the treatment of diabetes: potential promise of incretin-based therapies. J Am Soc Hypertens. 2009. 3:245–259.
44. Mannucci E, Rotella CM. Future perspectives on glucagon-like peptide-1, diabetes and cardiovascular risk. Nutr Metab Cardiovasc Dis. 2008. 18:639–645.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download