Abstract
Background
Long menstrual cycle is a risk factor for developing type 2 diabetes and cardiovascular disease in women. We aimed to evaluate the association between existing type 2 diabetes and oligomenorrhea before diagnosis of diabetes, and to observe the differences in this association among obese and non-obese Korean women.
Methods
Patients with type 2 diabetes (n=118) and without any clinical evidence of abnormal glucose regulation (n=258) who attended the outpatient clinic of a university hospital and were over age 30. Patients self-reporting a menstrual cycle over 40 days during their 20s were defined as oligomenorrhea before diagnosis of diabetes. Obesity was defined as having a body mass index (BMI) over 25 kg/m2.
Results
The frequency of oligomenorrhea before diagnosis of diabetes was almost two-fold higher in women with type 2 diabetes than in the control group (16.1% vs. 8.5%, P=0.03). Oligomenorrhea was associated with type 2 diabetes after adjusting for age, BMI, systolic blood pressure, triglycerides, and high density lipoprotein cholesterol (odds ratio, 3.89; 95% confidence interval, 1.37 to 11.04). Among women with oligomenorrhea before diagnosis of diabetes, the frequency of type 2 diabetes was significantly higher in obese subjects than in their non-obese counterparts (90.9% vs. 30.0%, P=0.03).
Irregular menstrual cycles are frequently associated with systemic diseases in women, and women with diabetes frequently have irregular menstrual cycles [1]. Several studies have shown that length of menstrual cycle is one of the risk factors for the development of type 2 diabetes [2-7]. Griffin et al. [4] reported that nearly 25% to 50% of young diabetic women of reproductive age suffer some form of menstrual dysfunction. A recent 6-year follow up in the Nurses' Health Study II (NHS II) reported an increased risk of developing type 2 diabetes in women with long (40 days or more) or irregular menstrual cycle between the ages of 18 to 22 years [5,6]. A cross-sectional study of women in Pima India also showed an increased frequency of type 2 diabetes in those women with a history of very long menstrual cycles [7].
Polycystic ovary syndrome (PCOS) is the most common cause of oligo- or amenorrhea in premenopausal women and most women with PCOS are insulin resistant [8]. It is an important risk factor for type 2 diabetes and the prevalence of women with impaired glucose tolerance is strikingly higher in this population than in their age comparable counterparts [9]. PCOS could reflect the potential association between risk for type 2 diabetes and menstrual irregularities.
In this study, we tried to evaluate the association between currently having type 2 diabetes and oligomenorrhea before diabetes was diagnosed. We also attempted to evaluate the differences in the association between oligomenorrhea and presence of type 2 diabetes among obese and non-obese Korean women.
We enrolled 376 non-pregnant premenopausal women who attended the Department of Endocrinology outpatient clinic or the health center at Ewha Womans University Mokdong Hospital from April 2005 to February 2006. Among the 376 women enrolled, 118 had type 2 diabetes and 258 acted as the control group. The women had presented for either an evaluation of a thyroid nodule or a routine health examination with no clinical evidence of glucose intolerance or personal history of type 2 diabetes. We excluded any subject with type 1 diabetes, previous gestational diabetes and/or family history of diabetes. The Institutional Review Board of Ewha Womans University Mokdong Hospital approved the study. Informed consent was obtained from all participants. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Participants completed a questionnaire about menstrual history including the number of days between menstrual cycles during adolescence, ages 20 to 29 and at present, current menstrual duration, age at menarche, history of severe teenage acne, hirsutism and lifestyle factors, such as regular exercise, smoking and alcohol consumption. Use of medications known to affect the menstrual cycle, including oral contraceptives, was also evaluated (Fig. 1). Anthropometric measurements were obtained, including body mass index (BMI) and waist circumference. Systolic and diastolic blood pressures were measured. Fasting serum glucose levels were measured using the glucose oxidase method and lipid profiles, including total cholesterol, high density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were measured using enzymatic methods. We categorized women as oligomenorrhea before the diagnosis of diabetes, if their menstrual cycle length during their 20s was over 40 days. We only enrolled subjects over 30 years old, since oligomenorrhea during a woman's 20s could be a phenotypic finding of diabetes.
We divided the subjects into obese (BMI ≥25 kg/m2) and non-obese (BMI <25 kg/m2) according to their present body weight.
Data were analyzed using SAS version 9.1 statistical software (SAS Institute, Cary, NC, USA). Because TG and HDL-C results showed slightly skewed distribution, analyses were performed using log-transformed data. Although mean values are shown for untransformed data, all P values are based on log-transformed data. Unadjusted associations between type 2 diabetes and potential risk factors were evaluated using the Student's t-test and χ2 test. Stratified analysis using the Cochran-Mantel-Haenszel test was performed to control for obesity as a confounding factor to the relationship between type 2 diabetes as an outcome variable and risk factors. Additionally, the Breslow-Day test for homogeneity was performed. Multiple logistic regression analyses were used to assess the independent contribution of oligomenorrhea during the participants' 20s to type 2 diabetes. Age, BMI, systolic blood pressure, TG, HDL-C, smoking, alcohol and exercise were used as covariates. All P values are two-tailed and statistical significance was defined as a P value less than 0.05.
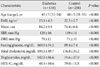
Women with type 2 diabetes were significantly older and more obese than the control subjects. The mean age was 45±5 years in women with type 2 diabetes and 40±5 years in the control subjects (P<0.001). Mean BMI and waist circumference were 25.1±4.3 kg/m2 and 84.2±9.9 cm in women with type 2 diabetes and 22.2±2.7 kg/m2 and 74.4±6.6 cm for the control group (P<0.001). Systolic blood pressure, fasting serum glucose, total cholesterol, and TG were also significantly higher and HDL-C was significantly lower in women with type 2 diabetes than in the control subjects (Table 1).
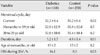
The menstrual history of women with type 2 diabetes and the control subjects is shown in Table 2. The current menstrual cycle was significantly longer in women with type 2 diabetes than in the control group (32.2±8.4 vs. 30.2±6.4 days, P=0.02). The frequency of oligomenorrhea from age 20 to 29 was almost two-fold higher in women with type 2 diabetes than in the control subjects (16.1% vs. 8.5%, P=0.03). The menstrual cycles during participants' adolescence and 20s was slightly longer in women with type 2 diabetes than in the control group; however, the difference was not statistically significantly.
Menstrual duration were significantly longer in participants with type 2 diabetes (5.2 vs. 4.9 days, P<0.01), but age at menarche did not differ significantly between the two groups. The frequency of oligomenorrhea before the diagnosis of diabetes or from age 20 to 29 was 18.9% for obese women with type 2 diabetes, 13.9% for non-obese women with type 2 diabetes and 8.5% for the control subjects (P for trend=0.01, Fig. 2). In multiple logistic regression analysis, oligomenorrhea was significantly associated with type 2 diabetes after adjusting for age, BMI, systolic blood pressure, TG, and HDL-C (odds ratio [OR], 3.89; 95% confidence interval [CI], 1.37 to 11.04; Table 3). The OR remained significant after the addition of lifestyle factors, such as regular exercise, smoking and alcohol consumption, as covariates (OR, 4.84; 95% CI, 1.64 to 14.28). We also compared the frequency of type 2 diabetes according to obesity and oligomenorrhea from age 20 to 29 (Table 4). Forty-one subjects had a past history of oligomenorrhea in their 20s. Among them, 11 were obese and 30 were non-obese. The frequency of type 2 diabetes was 90.9% (10 of 11) in obese women with a history of oligomenorrhea and 30.0% (9 of 30) in their non-obese counterparts. In contrast, 43 of 83 (51.8%) obese women without a history of oligomenorrhea and 56 of 252 (22.2%) non-obese women were diabetic patients. Additionally, when we performed the Cochran-Mantel-Haenszel stratified analysis to consider the possibility of an interaction between obesity, oligomenorrhea and diabetes, the OR of obesity X oligomenorrhea was 2.27 (95% CI, 1.11 to 4.66; P value for Breslow-Day test for homogeneity=0.09; data not shown). In obese subjects, diabetes was significantly associated with oligomenorrhea before and after adjusting for age and BMI, which was not replicated in the non-obese subjects (Table 4).
In this study, we observed that the frequency of oligomenorrhea during women's 20s was almost two-fold higher in women with type 2 diabetes than in the control subjects. The frequency was 18.9% in obese women with type 2 diabetes, 13.9% in non-obese type 2 diabetes and 8.5% in the control group.
Type 2 diabetes is a common endocrine disorder with high prevalence in women of reproductive age. Several studies have reported an increased prevalence of amenorrhea and other menstrual irregularities in diabetic women.
Having a long interlude between menstrual cycles is a risk factor of type 2 diabetes. Solomon et al. [5] reported that women with long (40 or more days) or highly irregular menstrual cycles had a significantly increased risk of developing type 2 diabetes in a 6-year follow-up study of the NHS II cohort. We observed that women with type 2 diabetes had significantly longer menstrual cycles than the control group (32.52±8.4 vs. 30.2±6.4 days, P=0.02), and the frequency of oligomenorrhea in their 20s was almost two-fold higher in women with type 2 diabetes than in the control subjects. This association was also seen in the multiple logistic regression analysis, after adjusting for age, obesity and various lifestyle habits, such as regular exercise, smoking, and alcohol consumption.
Irregular menstrual cycle and type 2 diabetes share common risk factors such as obesity, hyperinsulinemia and a more central distribution of fat [10,11]. Obesity is related to the increased peripheral conversion of androgens to estrogens in the adipose tissue [12,13]. This becomes a major risk factor for type 2 diabetes, associated with infertility and menstrual abnormalities [7]. Higher estrogen concentrations result in menstrual abnormalities and anovulation by negative feedback at the hypothalamo-pituitary level [14]. In our data, the association between menstrual irregularity and type 2 diabetes was strongest in women with a BMI greater than 25 kg/m2, demonstrating that obesity increases the risk of type 2 diabetes and menstrual irregularity.
Insulin resistance and hyperinsulinemia are also associated with having an irregular menstrual cycle. The mechanism of insulin action on the ovaries leads to hyperinsulinemia resulting in excessive androgen production, leading to menstrual cycle disturbances and decreased fertility rates [14]. Insulin resistance and the associated beta cell dysfunction predispose a person to type 2 diabetes and high insulin levels often predict the progression to type 2 diabetes [15]. Hyperinsulinemia seems to synergize with pituitary gonadotropins to stimulate ovarian theca cell androgen production, exacerbating the insulin resistance [16]. Elevated testosterone levels have also been reported as the cause for oligomenorrhea in women without clinical hyperandrogenism [17].
Hyperinsulinemia inhibits sex hormone-binding globulin (SHBG) production by the liver [18], and is associated with low SHBG concentrations, which leads to higher free testosterone concentrations [19]. Low SHBG concentration is also a risk factor for type 2 diabetes in women [20].
PCOS is characterized by anovulation, androgen excess and insulin resistance [8]. Many women with PCOS are insulin resistant and approximately 20% of obese women with PCOS have impaired glucose tolerance [21]. Studies of adolescent girls with PCOS show that insulin resistance is present early in the course of the syndrome [22-24]. The high prevalence of these risk factors among adolescent girls with PCOS puts them at an increased risk for developing type 2 diabetes [25]. In women with PCOS, hyperinsulinemia contributes to hyperandrogenism by increasing ovarian androgen production and decreasing SHBG [26]. A decrease in plasma insulin results in an increase in SHBG with a concurrent decrease in free testosterone, and these changes are consistent with the concept that insulin is a direct negative regulator of hepatic SHBG production [27-29].
There are some limitations to our study. One limitation is that the small sample size does not allow for the generalization of our results. Secondly, it is possible that while there may be an interaction between obesity, oligomenorrhea and diabetes, obesity could be a confounding factor. However, we adjusted for BMI in both the obese and non-obese groups and the OR remained markedly higher in obese subjects, as compared to the non-obese subjects. Thirdly, we did not measure testosterone levels and hirsutism scores in all of the participants. Therefore, there may have been women with undiagnosed PCOS in the diabetic group, which could have affected the results. Obesity could have also been a confounding factor in the diabetic group, however, we did not ask about any weight change from age 20 to current age. Finally, there could be a recall bias. Since women in our study self-reported any history of long or irregular menstrual cycles during their adolescence and from age 20 to 29, recall bias could have interfered with the study results. Studies on validity and reliability of recall of early menstrual history have shown limitations in accuracy and precision [30-33]. However, studies examining menstrual data as an exposure measure continue to rely on retrospective data, given the expense and subject burden associated with gathering this data prospectively. In conclusion, we observed an association between women who currently have type 2 diabetes and having had a long menstrual cycle before being diagnosed as type 2 diabetic. Long menstrual cycles may be an important risk factor for the development of type 2 diabetes in women. However, prospective studies will be required to assess the causality.
Figures and Tables
Fig. 2
Frequency of oligomenorrhea from age 20 to 29 in obese and non-obese women with type 2 diabetes and controls.

Table 3
Multiple logistic regression analysis for the risk of type 2 diabetes

Triglycerides were analyzed after log transformation. Covariates were age, BMI, SBP, triglycerides, and HDL-C.
OR, odds ratio; CI, confidence interval; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high density lipoprotein cholesterol; OM, oligomenorrhea.
aOM was defined as a menstrual cycle >40 days during the participant's 20s.
References
1. Edelman SV. Type II diabetes mellitus. Adv Intern Med. 1998. 43:449–500.
2. Knowler WC, Saad MF, Pettitt DJ, Nelson RG, Bennett PH. Determinants of diabetes mellitus in the Pima Indians. Diabetes Care. 1993. 16:216–227.
3. Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC, Speizer FE, Manson JE. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002. 87:2013–2017.
4. Griffin ML, South SA, Yankov VI, Booth RA Jr, Asplin CM, Veldhuis JD, Evans WS. Insulin-dependent diabetes mellitus and menstrual dysfunction. Ann Med. 1994. 26:331–340.
5. Solomon CG, Rich-Edwards JW, Dunaif A, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, Spiegelman D, Manson JE. Abnormal menstrual cycle length predicts subsequent non-insulin-dependent diabetes mellitus. Am J Epidemiol. 1998. 147:11 Suppl. S60.
6. Solomon CG, Hu FB, Dunaif A, Rich-Edwards J, Willett WC, Hunter DJ, Colditz GA, Speizer FE, Manson JE. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. JAMA. 2001. 286:2421–2426.
7. Roumain J, Charles MA, de Courten MP, Hanson RL, Brodie TD, Pettitt DJ, Knowler WC. The relationship of menstrual irregularity to type 2 diabetes in Pima Indian women. Diabetes Care. 1998. 21:346–349.
8. Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose intolerance in polycystic ovary syndrome: a position statement of the Androgen Excess Society. J Clin Endocrinol Metab. 2007. 92:4546–4556.
9. Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999. 84:165–169.
10. Hartz AJ, Barboriak PN, Wong A, Katayama KP, Rimm AA. The association of obesity with infertility and related menstural abnormalities in women. Int J Obes. 1979. 3:57–73.
11. Dunaif A. Hyperandrogenic anovulation (PCOS): a unique disorder of insulin action associated with an increased risk of non-insulin-dependent diabetes mellitus. Am J Med. 1995. 98(1A):33S–39S.
12. Longcope C, Baker R, Johnston CC Jr. Androgen and estrogen metabolism: relationship to obesity. Metabolism. 1986. 35:235–237.
13. Weiss DJ, Charles MA, Dunaif A, Prior DE, Lillioja S, Knowler WC, Herman WH. Hyperinsulinemia is associated with menstrual irregularity and altered serum androgens in Pima Indian women. Metabolism. 1994. 43:803–807.
14. Poretsky L, Kalin MF. The gonadotropic function of insulin. Endocr Rev. 1987. 8:132–141.
15. Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus: prospective studies of Pima Indians. N Engl J Med. 1993. 329:1988–1992.
16. Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999. 22:141–146.
17. Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed). 1986. 293:355–359.
18. Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. 1988. 67:460–464.
19. Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, Clore JN, Blackard WG. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991. 72:83–89.
20. Haffner SM, Valdez RA, Morales PA, Hazuda HP, Stern MP. Decreased sex hormone-binding globulin predicts noninsulin-dependent diabetes mellitus in women but not in men. J Clin Endocrinol Metab. 1993. 77:56–60.
21. Dunaif A, Finegood DT. Beta-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1996. 81:942–947.
22. Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006. 91:492–497.
23. Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001. 138:38–44.
24. Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002. 87:1017–1023.
25. Tfayli H, Arslanian S. Menstrual health and the metabolic syndrome in adolescents. Ann N Y Acad Sci. 2008. 1135:85–94.
26. Hull MG. Epidemiology of infertility and polycystic ovarian disease: endocrinological and demographic studies. Gynecol Endocrinol. 1987. 1:235–245.
27. Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab. 1987. 65:499–507.
28. Ireland K, Child T. Polycystic ovary syndrome and the postmenopausal woman. J Br Menopause Soc. 2006. 12:143–148.
29. Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1983. 57:356–359.
30. Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, Rand WM. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol. 2002. 155:672–679.
31. Jukic AM, Weinberg CR, Wilcox AJ, McConnaughey DR, Hornsby P, Baird DD. Accuracy of reporting of menstrual cycle length. Am J Epidemiol. 2008. 167:25–33.
32. Bachand AM, Cragin LA, Reif JS. Reliability of retrospectively assessed categorical menstrual cycle length data. Ann Epidemiol. 2009. 19:501–503.
33. Small CM, Manatunga AK, Marcus M. Validity of self-reported menstrual cycle length. Ann Epidemiol. 2007. 17:163–170.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download