INTRODUCTION
Many patients with type 2 diabetes have a low concentration of high density lipoprotein cholesterol (HDL-C). The fact that a low level of HDL-C is a predictor of cardiovascular disease, it follows that a low level of HDL-C in patients with type 2 diabetes may contribute to the increased cardiovascular risk associated with this condition. The precise cause of the low HDL-C in type 2 diabetes is not known but may be the consequence of insulin resistance, augmented very low density lipoprotein production and increased activities of cholesteryl ester transfer protein (CETP) and endothelial lipase. Recent studies showing that HDLs have the ability to improve increase the uptake of glucose by skeletal muscle [1] and to stimulate the secretion of insulin from pancreatic beta cells [2] raise the possibility that the low HDL concentration in type 2 diabetes may also contribute to a worsening of diabetic control or, indeed, to the progression of prediabetes to the full diabetic state. Therefore, a low concentration of HDLs may not only be a consequence of the diabetes and a contributor to the increased cardiovascular risk in diabetics but may also result in a worsening of glycemic control in people with type 2 diabetes.
POSSIBLE MECHANISMS RESPONSIBLE FOR A LOW HDL-C IN DIABETES
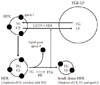
The typical dyslipidemia in type 2 diabetes includes an elevated level of plasma triglyceride, a low level of HDL-C and a low density lipoprotein (LDL) fraction that is characterized by small dense particles [3]. The low level of HDL-C in these patients is associated with the presence of HDL particles are also smaller and denser than normal. Since it is known that the particle size of HDLs correlates inversely with the concentration of plasma triglyceride, the low level of HDL-C associated with diabetes may be secondary to an elevated level of plasma triglyceride. This inverse relationship between the concentrations of HDL-C and plasma triglyceride is the consequence of an increased rate of HDL catabolism that may be secondary to triglyceride enrichment of HDL particles in a mechanism dependent on the activities of CETP and hepatic lipase [4] as outlined in Fig. 1.
CARDIO-PROTECTIVE PROPERTIES OF HDLS
HDLs have a number of properties with the potential to protect against cardiovascular disease (Table 1) [5]. The best known of these relates to the ability of HDLs to promote the efflux of cholesterol from macrophages in the artery wall. However, HDLs have several additional properties with the potential to protect against the consequences of atherosclerotic vascular disease. These include anti-oxidant, anti-thrombotic and anti-inflammatory effects and the ability to promote both the function and repair of the endothelium. Some of the potentially protective functions of HDLs may be compromised in patients with diabetes. There is also evidence that HDLs have the capacity to improve diabetic control and possibly to protect against the development of type 2 diabetes [2].
HDL FUNCTION IS COMPROMISED IN DIABETES
Apolipoprotein (apo) A-I (the major HDL apolipoprotein) may become glycated in people with diabetes. Such glycation has been reported to impair both the ability of HDLs to promote cholesterol efflux from macrophages [6] and their ability to inhibit vascular inflammation [7]. This finding is of potential patho-physiological significance given that subjects with type 2 diabetes, especially those with micro- and macro-vascular complications, tend to be in a pro-inflammatory state. This highlights the importance of maintaining good glycemic control in people with diabetes and raises the possibility that therapeutic intervention with cross-link breakers, which reportedly prevent protein modifications, may have the potential to decrease the risk of the microvascular, and possibly the macrovascular complications that accompany diabetes. It is of interest to note that the endothelial vasoprotective effects of HDLs are impaired in patients with type 2 diabetes [8]. However, when diabetic subjects are treated with niacin, the endothelial protective properties of HDLs are restored [8].
EFFECTS OF HDLS ON GLUCOSE HOMEOSTASIS
There is a growing body of experimental evidence that HDLs have beneficial effects on glucose homeostasis by both increasing pancreatic beta cell function and by enhancing plasma glucose disposal.
HDLs and pancreatic beta cell function
The ATP binding cassette transporter A1 (ABCA1) promotes efflux of cholesterol from cells to lipid-free/lipid-poor apoA-I in the extracellular space. It has been suggested that ABCA1 deletion results in cholesterol accumulation in the cell membrane of beta cells, with a subsequent inhibition of the exocytosis of insulin from secretory granules, and inhibition of insulin secretion [9]. It has also been reported that HDLs have beneficial effects on beta cells by inhibiting apoptosis [10]. And more recently, it has been shown in studies conducted in vitro using both Min6 cells and primary islets that HDLs isolated from human plasma and also the major HDL proteins, apoA-I and apoA-II, increase insulin synthesis and secretion up to 5-fold [2].
HDL and muscle glucose uptake
The observation that HDLs increase cellular glucose uptake in cultures of primary human skeletal muscle cells isolated from patients with type 2 diabetes mellitus [1] adds further support to the view that HDLs have the capacity to improve diabetic control (and possibly delay the development of new onset diabetes) by several mechanisms.
STRATEGIES FOR RAISING HDL-C IN PATIENTS WITH TYPE 2 DIABETES
Lifestyle
Weight reduction
Most overweight people have a low level of HDL-C. Furthermore, weight reduction is usually accompanied by an increase in the HDL-C level, although to be effective, the weight loss needs to be substantial and sustained. The mechanism underlying a relationship between body weight and HDL-C concentration is uncertain. However, the fact that most patients with type 2 diabetes are overweight provides a strong basis for recommending weight reduction as a strategy to raise the level of HDL-C in such patients.
Physical activity
High levels of aerobic activity are associated with high levels of HDL-C. Furthermore, increasing the level of physical activity in people with low levels of HDL-C, especially in those who are overweight, increases the HDL-C concentration. An exercise-induced increase in the level of HDL-C may be secondary to an increased activity of lipoprotein lipase and the consequent reduction in concentration of plasma triglyceride. It has been argued that the single most important preventable cause of low HDL-C in the modern world is a low level of physical activity.
A recent meta-analysis has confirmed the benefit of regular aerobic exercise on raising HDL-C levels and provided some insights into how much exercise is required [11]. The analysis included 25 randomized controlled studies that were designed to evaluate the effect of exercise training on HDL-C levels. Overall, the mean exercise-induced increase in HDL-C was 2.53 mg/dL (P<0.001). Importantly, an increase in HDL-C concentration was apparent only in people who expended at least 900 kcal or exercised for at least 120 minutes each week. In these people, every 10 minutes prolongation of exercise per session was associated with a 1.4 mg/dL increase in HDL-C. In further analyses it was found that the increase in HDL-C was greatest in people whose body mass index was <28 kg/m2.
These findings re-enforce recommendations for increasing levels of activity as a cardio-protective strategy in people with type 2 diabetes.
Alcohol consumption
Alcohol consumption increases the level of HDL-C, possibly secondary to an inhibition of CETP. However, it should be emphasized that it is not known whether the HDL-C elevation associated with alcohol consumption is cardio-protective.
Smoking cessation
Smoking reduces the concentration of HDL-C and smoking cessation is associated with an up to 10% increase in HDL-C level. The mechanism by which smoking reduces the level of HDL-C is not known.
Pharmacological management
Levels of HDL-C are increased by treatment with several classes of currently available lipid-modifying agents. These include fibrates, statins, and (especially) niacin, with evidence accumulating that such increases do translate into a reduced risk of cardiovascular disease.
Fibrates
The ability of fibrates to increase in concentration of HDL-C in patients with type 2 diabetes is rather modest, with increases of only 2% to 3% reported in two large clinical trials [12,13]. But treatment with fibrates (including gemfibrozil, bezafibrate, and fenofibrate) is highly effective in reducing coronary risk in people with the combination of low HDL-C and elevated triglyceride [14]. In contrast, people with normal levels of HDL-C and triglyceride levels appear to derive little macro-vascular benefit from treatment with fibrates. These results have been observed in both diabetic and non-diabetic subjects. However, the cardiovascular benefits of fibrates in people with low HDL-C and high triglyceride are largely unrelated to fibrate-induced changes in either HDL-C or triglyceride. This suggests that a dyslipidemia characterized by a low HDL-C and high triglyceride identifies a group of people who derive substantial benefit from treatment with a fibrate but that the benefit is by a mechanism other than by changes in lipid concentrations.
Statins
Statins raise the level of HDL-C by 3% to 15%. The increase is greatest in those in whom the baseline level of HDL-C is low [15]. Given that the level of HDL-C tends to be low in patients with type 2 diabetes, it might be expected that the HDL-C increase induced by a statin would be greater in diabetics than in non-diabetics. The reality is the opposite, with evidence that the increase in level of HDL-C following treatment with a statin is much less in diabetic than in non-diabetic patients [15]. The explanation and the clinical implications of a reduced HDL-C response to statins in people with diabetes is not known.
Statins have been reported to have an adverse effect on glucose homeostasis, including a possible increase in development of new onset diabetes [16]. The mechanism is not known. The cholesterol content of pancreatic beta islet cells does impact of insulin synthesis and secretion with evidence that an increase in cell cholesterol content decreases insulin secretion [17]. However, given that statin treatment will, if anything, decrease cell cholesterol levels, this is an unlikely explanation for the observed increase in new onset diabetes in people treated with statins. It is possible that statins decrease insulin sensitivity in the liver or muscle, although there is no direct experimental evidence to support this. Thus, it is currently not known why statins impact adversely on glucose homeostasis. However, given the strong evidence that statins markedly reduce cardiovascular risk patients with type 2 diabetes [18], the clinical impact of a small increase in new onset diabetes is probably very small.
Niacin
Niacin increases HDL-C by up to 35% [19]. In addition, treatment with niacin is associated with a change in the subpopulation distribution of HDLs towards larger particles [20], a change predictive of both a slowing of coronary disease progression and a reduction in cardiovascular events.
The precise mechanism by which niacin increases the concentration of HDL-C is not known, although there is evidence that it delays the catabolism of HDL particles, possibly by decreasing activity of CETP. There is also evidence that niacin increases the synthesis of apoA-I.
In addition to increasing the plasma concentration of HDL-C and apoA-I, niacin has the capacity to modify HDLs in such a way that their function is enhanced. In one study HDLs were isolated from patients with type 2 diabetes and compared with HDLs isolated from healthy subjects [8]. Effects of the isolated HDLs on endothelium-dependent vasodilation and early endothelial progenitor cell-mediated endothelial repair were measured. Whereas the HDLs from healthy subjects stimulated endothelial nitric oxide production, reduced endothelial oxidant stress and improved both endothelium-dependent vasodilation and early endothelial progenitor cell-mediated endothelial repair, these beneficial endothelial effects of HDL were not observed in the HDLs isolated from diabetic patients. However, after the diabetic patients had been treated for 3 months with niacin, the ability of the isolated HDLs to stimulate endothelial nitric oxide, to reduce superoxide production and to promote endothelial progenitor cell-mediated endothelial repair were greatly improved [8].
Niacin has also been reported both to enhance the anti-inflammatory effects of HDLs [21] and to inhibit vascular inflammation by a mechanism that appears to be unrelated to changes in plasma lipids [22].
There is mounting evidence that treatment with niacin reduces cardiovascular events and promotes regression of atherosclerosis [23] as revealed by imaging studies in human, with emerging evidence that the benefit is related (at least in part) to the magnitude of the increase in HDL-C.
If niacin is so effective, why is it not more widely used? The original, immediate release forms of niacin caused severe flushing that resulted in many people stopping the medication. And those who did take it were often unable to achieve the recommended therapeutic dose of 2 g per day. The flushing problem has been substantially reduced by the development of newer extended release formulations of niacin. Not only is the extended release form better tolerated but it may be taken as a single daily dose rather than in three divided doses as was the case with the immediate release form of the drug [24]. Flushing is reduced even more when an extended release form of niacin is combined with the anti-flushing agent, laropiprant, allowing more patients to take the drug at its recommended dose of 2 g per day [25]. The mechanism by which niacin causes flushing is understood. It binds to a receptor in the skin that leads to the production of PGD2. PGD2 then binds to the DP1 receptor causing dilation of the blood vessels in the skin leading to flushing. Laropiprant blocks the DP1 receptor and thus inhibits the flushing caused by niacin
Like statins, niacin is also known to have adverse effects on glucose homeostasis by decreasing insulin sensitivity and possibly worsening diabetic control. Whether the effects of niacin on glucose homeostasis are of clinical importance is uncertain but will be determined by the results of ongoing cardiovascular clinical outcome trials with niacin.
CONCLUSION
Many patients with type 2 diabetes have a low plasma concentration of HDL-C that may contribute to an increased risk of developing cardiovascular disease. The observation that HDLs have beneficial effects on pancreatic beta cell function and glucose uptake by skeletal muscle, adds support to the proposition that HDL-raising in people with type 2 diabetes may be anti-atherogenic as a consequence of both direct effects on the artery wall and also by improving diabetic control. It will be of great interest to see whether studies with HDL raising agents such as CETP inhibitors currently under investigation in clinical trials have beneficial effects on diabetic control.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download