This article has been
cited by other articles in ScienceCentral.
Abstract
Hemangioma of the adrenal gland is a rare benign tumor. The diagnosis is often postoperative on histopathological examination with the presence of blood filled, dilated vascular channels. Approximately 60 surgical cases have been reported in the literature so far. We present a case of a 41 years old man who was incidentally found to have a 5 cm right adrenal mass, on abdominal ultrasonography done to evaluate for right renal colicky pain. Contrast enhanced computed tomography (CT) of the abdomen revealed a 5 cm right adrenal lesion with peripheral enhancement on arterial phase with central necrosis consistent with a pheochromocytoma. Laparoscopic adrenalectomy (LA) was performed through a transperitoneal flank approach. Pathological examination revealed a 6 cm adrenal mass with extensive central necrotic areas mixed with dilated vascular channels with the diagnosis of adrenal hemangioma. Adrenal hemangiomas are extremely uncommon. They are mostly incidentally diagnosed owing to their non-secretory nature with non-specific symptom profile. LA is technically safe and feasible for large adrenal tumors, but controversy exists in cases of suspected malignancy. We prefer laparoscopic approach on the basis of preoperative abdominal CT scan that excludes radiological signs of adrenocortical carcinoma such as peri-adrenal infiltration and vascular invasion. LA is considered the standard treatment in case of diagnosis of benign lesions. The most important thing preoperatively is to work out the risk of malignancy and hormonal profile of the patient before going for the excision of the adrenal incidentaloma.
Keywords: Laparoscopic adrenalectomy, Adrenal hemangioma, Adrenal incidentaloma, Pheochromocytoma
INTRODUCTION
An adrenal incidentaloma (AI) is a mass lesion greater than 1 cm in diameter, incidentally revealed by radiologic examination (
1). Adrenal masses are often discovered after an imaging procedure is performed for another complain, not suspecting adrenal disease. The differential diagnosis of AIs includes many primary, metastatic, benign, and malignant entities. Once discovered, 2 main questions need to be answered that are crucial to the management of AIs. Is it malignant? Is it functioning? The majority of AIs are clinically non-functioning benign adrenocortical adenomas (
2). Other commonly reported diagnoses include cortisol-secreting adrenocortical adenoma, pheochromocytoma, adrenocortical carcinoma, and metastatic carcinoma (
1). Autopsy studies suggest a prevalence of clinically unapparent adrenal masses of around 2% (range, 1.0%–8.7%), which increases with age (
234). Radiological studies report a frequency of around 3% in the age of 50 years, which increases up to 10% in the elderly (
234). Adrenal hemangiomas are rare non-functioning benign tumors. The diagnosis is often postoperative on histological exam with the presence of blood filled, dilated vascular spaces. Approximately 60 surgical cases have been reported in the literature so far.
CASE REPORT
We report a case of a 41 years old male who presented to his local physician for right renal colicky pain. He had history of renal stones, analgesics were prescribed and a routine ultrasonography (USG) was performed which revealed a right renal calculus along with a round heterogeneous right suprarenal mass. Abdominal contrast enhanced computed tomography (CT) was performed for detailed evaluation which revealed a 5 cm right adrenal lesion with peripheral enhancement on arterial phase with central necrosis consistent with a pheochromocytoma (
Fig. 1). The left adrenal gland was normal and there were no others significant radiologic findings. He was then referred to our institution for further management. He reported no complains of headache, sweating, palpitations, weight gain or loss. He was normotensive with normal blood sugar levels. The patient did not have clinical features suggestive of a functioning adrenal tumor, but we performed complete hormonal tests as per the department protocol, the results of which are listed below in
Table 1.
Fig. 1
CT of abdomen. (A) A non-contrast enhanced CT scan showing a large encapsulated right adrenal lesion with regular margin. (B, C) Contrast enhanced CT images obtained in arterial, venous phase, showing a hypodense centre with peripheral enhancement.
CT = computed tomography.

Table 1
Hormonal profile of the patient

|
Test |
Patient value |
Normal reference value |
|
Plasma free nor metanehprine (pg/mL) |
106.10 |
0.00–196.00 |
|
Plasma free metanehprine (pg/mL) |
12.90 |
0.00–65.00 |
|
Cortisol (8 am; µg/dL) |
12.42 |
5.00–23.00 |
|
Cortisol (4 pm; µg/dL) |
7.22 |
3.00–16.00 |
|
24-hour urinary VMA |
1.26 mg/24 hr |
0.00–15.00 mg/24 hr |
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography and metaiodobenzylguanidine (MIBG) scintigraphy revealed no FDG or MIBG concentrating lesion in the whole body survey including the right adrenal lesion. Because malignancy could not be excluded, the patient was taken up for surgery for non-functioning pheochromocytoma.
Laparoscopic adrenalectomy (LA) was performed by a transperitoneal flank approach in left lateral decubitus position. Open technique was used to insert 10 mm camera port about 2.5 cm superior and right lateral to the umbilicus and pneumoperitoneum was created. Three other ports were placed in the right subcostal region. Omental adhesions to liver were divided and liver retraction revealed the adrenal mass. Dissection was carried out using harmonic scalpel. Short adrenal vein which was identified along the lateral border of inferior vena cava entering the right adrenal on superior and medial aspect, clipped and divided. Medial to lateral and inferior to superior, the gland was mobilized until it was completely free. The specimen was retrieved in an endo bag and delivered out after extending the camera port incision. Operative time was one hour and blood loss was 30 mL. Patient was started on orals on post-operative day (POD) zero and shifted to full diet next day and discharged on third POD. Pathological examination of the specimen revealed a 6.0×5.5×2.5 cm adrenal mass (
Fig. 2). Cut section showed a well circumscribed thinly encapsulated tumor, brownish in color with few areas of haemorrhage. Adrenal parenchyma identified at the periphery measuring 2.5×1.0×1.0 cm. Microscopically it was an adrenal neoplasm with features consistent with hemangioma. It was composed of vascular channels of various sizes from capillary size to large size, with an endothelial lining (
Fig. 3), which were immunohistochemically strongly positive for factor CD34 and Vimentin. The vessels showed red blood cells in the lumen and occasional vessels showed thrombus formation (
Fig. 4). Normal adrenal cortex and medulla seen at periphery. There was no evidence of malignancy (
Fig. 5).
Fig. 2
Adrenalectomy specimen: 6.0×5.5×2.5 cm gross adrenal specimen.
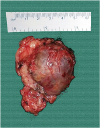
Fig. 3
Light microscopic image of adrenal hemangioma. Blood filled dilated vascular channels (hematoxylin and eosin staining, ×40).
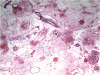
Fig. 4
Light microscopic image of adrenal hemangioma. Multiple dilated interconnecting vascular channels with thrombosis (hematoxylin and eosin staining, ×40).
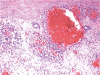
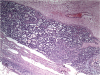
Fig. 5
Light microscopic image of adrenal hemangioma. Residual adrenal parenchyma can be seen at the periphery (hematoxylin and eosin staining, ×40).
DISCUSSION
An AI is a mass lesion greater than 1 cm in diameter, incidentally revealed by radiologic examination not performed for suspected adrenal disease (
1). The widespread use of abdominal USG, CT, and magnetic resonance imaging (MRI) has resulted in an increased number of incidental findings of adrenal masses. The most frequent AIs are non-functioning cortical adenomas accounting for almost 75% of the adrenal masses (
35). However, other diagnosis included functioning cortical adenomas (Cushing's syndrome, Conn's disease), pheochromocytoma, adrenal metastases, cystic lesions, myelolipomas. The first important step after detection is the evaluation for the possibility of malignancy and subclinical hormonal hyperfunction.
Clinical practice guidelines for AI management issued in 2016 by the European Society of Endocrinology, in collaboration with the European Network for the Study of Adrenal Tumors (ENSAT) were used to evaluate our patient (
6). CT imaging reported a well-defined hypodense adrenal lesion measuring 5.0×4.0×5.1 cm with peripheral enhancement on arterial phase consistent with a pheochromocytoma. Biochemical evaluation revealed a non-functioning tumor. LA is the standard of care for unilateral adrenal tumors with clinically significant hormone excess and is suggested for patients with unilateral adrenal masses with radiological findings suspicious of malignancy and a diameter ≤6 cm, but without evidence of local invasion (
6). The indication for excision is based on tumor size. AIs larger than 6 cm in diameter must be excised because of the risk of adrenal malignancy. For lesions measuring 4 to 6 cm, other imaging features, a history of extra-adrenal malignancy, patient preference should be taken into consideration. Our patient underwent LA with the working diagnosis of a non-functioning pheochromocytoma. Postoperative histopathological examination of the specimen was reported as an adrenal hemangioma.
Hemangiomas of the adrenal gland are fairly uncommon. Due to the low occurrence and the absence of specific symptoms, the majority of adrenal hemangiomas are diagnosed after surgical resection. Usually, adrenal hemangioma is unilateral, being more frequent in the sixth or seventh decade of life with a male-female ratio being 1:2 (
7). They are mostly asymptomatic but when they have a large size, the symptoms would include flank pain and may even be palpable on physical examination. Though there are no characteristic signs on radiological studies, contrast enhanced CT scan can suggest the presence of hemangioma when showing a mass with heterogeneous enhanced areas in a centripetal pattern. Tumor calcifications can be found and they are attributed to multiple phleboliths inside the dilated vascular spaces (
8). In almost all previously published cases of adrenal hemangioma, the reason for surgical excision was tumor size. The presence of tumor functionality and associated malignancy were less frequent indications for surgical resection. Although the preoperative diagnosis of adrenal hemangioma is difficult, it is an important differential diagnosis as 2 cases have been reported where large adrenal hemangiomas presented with spontaneous life threatening retroperitoneal haemorrhage (
910). Upon identification of an AI, the aim should be the establish a definitive diagnosis. The patient should be simultaneously assessed for the potential risk of malignancy and hormonal activity of the tumor. Surgery is recommended for all hormonally active adrenal tumors, for large adrenal tumors, symptomatic and tumors with probable malignancy. LA is considered the standard treatment for benign lesions greater that 4 cm in largest diameter. In presence of local invasion or vascular infiltration LA may be converted to the open technique. Patient factors and preferences, hormonal studies, radiologic characteristics, and the surgeon's expertise help to decide the correct line of management for patients incidentally detected to have adrenal mass.
ACKNOWLEDGMENTS
We would like to thank Dean of our institute for the use of hospital records for research and publications.
References
1. Young WF Jr. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601–610.
2. Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004; 25:309–340.

3. Grumbach MM, Biller BM, Braunstein GD, Campbell KK, Carney JA, Godley PA, et al. Management of the clinically inapparent adrenal mass (“incidentaloma”). Ann Intern Med. 2003; 138:424–429.

4. Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev. 1995; 16:460–484.

5. Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003; 149:273–285.

6. Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology clinical practice guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016; 175:G1–G34.

7. Salup R, Finegold R, Borochovitz D, Boehnke M, Posner M. Cavernous hemangioma of the adrenal gland. J Urol. 1992; 147:110–112.

8. Thiele JW, Bodie B. Adrenal hemangioma. Surgery. 2001; 129:373–374.

9. Forbes TL. Retroperitoneal hemorrhage secondary to a ruptured cavernous hemangioma. Can J Surg. 2005; 48:78–79.
10. Boraschi P, Campatelli A, Di Vito A, Perri G. Hemorrhage in cavernous hemangioma of the adrenal gland: US, CT and MRI appearances with pathologic correlation. Eur J Radiol. 1995; 21:41–43.









