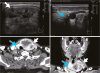
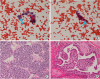
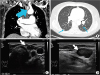
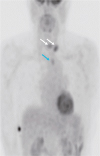
1. Sakamoto A, Kasai N, Sugano H. Poorly differentiated carcinoma of the thyroid. A clinicopathologic entity for a high-risk group of papillary and follicular carcinomas. Cancer. 1983; 52:1849–1855.

2. Bongiovanni M, Sadow PM, Faquin WC. Poorly differentiated thyroid carcinoma: a cytologic-histologic review. Adv Anat Pathol. 2009; 16:283–289.
3. Volante M, Landolfi S, Chiusa L, Palestini N, Motta M, Codegone A, et al. Poorly differentiated carcinomas of the thyroid with trabecular, insular, and solid patterns: a clinicopathologic study of 183 patients. Cancer. 2004; 100:950–957.

4. Wreesmann VB, Ghossein RA, Patel SG, Harris CP, Schnaser EA, Shaha AR, et al. Genome-wide appraisal of thyroid cancer progression. Am J Pathol. 2002; 161:1549–1556.

5. Luna-Ortiz K, Hurtado-López LM, Domínguez-Malagón H, Ramírez-Marín R, Zaldivar-Ramírez FR, Herrera-Gomez A, et al. Clinical course of insular thyroid carcinoma. Med Sci Monit. 2004; 10:CR108–CR111.
6. Pulcrano M, Boukheris H, Talbot M, Caillou B, Dupuy C, Virion A, et al. Poorly differentiated follicular thyroid carcinoma: prognostic factors and relevance of histological classification. Thyroid. 2007; 17:639–646.

7. Patel KN, Shaha AR. Poorly differentiated thyroid cancer. Curr Opin Otolaryngol Head Neck Surg. 2014; 22:121–126.

8. Onenerk M, Canberk S, Gunes P, Erkan M, Kilicoglu GZ. Oncocytic variant of poorly differentiated thyroid carcinoma: “Is diagnosis possible by fine-needle aspiration?”. Cytojournal. 2016; 13:23.

9. Rocha AS, Soares P, Fonseca E, Cameselle-Teijeiro J, Oliveira MC, Sobrinho-Simões M. E-cadherin loss rather than β‐catenin alterations is a common feature of poorly differentiated thyroid carcinomas. Histopathology. 2003; 42:580–587.

10. Kane SV, Sharma TP. Cytologic diagnostic approach to poorly differentiated thyroid carcinoma: a single-institution study. Cancer Cytopathol. 2015; 123:82–91.

11. Ibrahimpasic T, Ghossein R, Carlson DL, Nixon I, Palmer FL, Shaha AR, et al. Outcomes in patients with poorly differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2014; 99:1245–1252.

12. Sobrinho-Simões M, Sambade C, Fonseca E, Soares P. Poorly differentiated carcinomas of the thyroid gland: a review of the clinicopathologic features of a series of 28 cases of a heterogeneous, clinically aggressive group of thyroid tumors. Int J Surg Pathol. 2002; 10:123–131.
13. Seoung HG, Kim JH, Choi JC, Kim SM, Kim SS, Kim BH, et al. A case of papillary thyroid cancer recurring as an esophageal submucosal tumor. Chonnam Med J. 2012; 48:60–64.

14. Lee MY, Kim SE, Kim HC, Han SH, Shin DH, Kim DH, et al. Direct invasion of thyroid papillary carcinoma to esophagus presenting as an intraluminal polypoid mass which causes hematemesis. Korean J Gastrointest Endosc. 2001; 23:466–469.
15. Muñoz de Nova JL, Dworzynska A, Lorente-Poch L, Sancho JJ, Sitges-Serra A. Esophageal recurrence of medullary thyroid carcinoma. Gland Surg. 2015; 4:564–566.
16. Lee B, Cook G, John L, Harrington K, Nutting C. Follicular thyroid carcinoma metastasis to the esophagus detected by 18FDG PET/CT. Thyroid. 2008; 18:267–271.