Abstract
Mycoplasma genitalium (MG) is the smallest self-replicating bacterium. Although small in size, unique MG genome induces distinctive and often serious characteristics in the infected cells. Due to its small genome and chronic symptomatic characteristics in the infected host, it first appears as a weak, insignificant, and easily controllable microbe. However, it is not a monotonous chrysalis, but rather a multicolored butterfly with various capabilities. Repetitive DNA sequence in MG's immunodominant MgPa operon has been considered as an efficient strategy to evade the host immune surveillance and mediate MG's genetic flexibility. Because of MG's pathogenicity in multiple organs, various antimicrobials are prescribed, further exerting selection pressure on microbes. Consequently, a rapidly increasing drug resistance in macrolide and moxifloxacin has been frequently reported globally, radically decreasing the overall cure rate of infection. Re-infection can be defined as a new MG infection through antigenic variation, while persistent infection refers to recurrent infections caused by the same MG isolate through acquisition of antimicrobial resistance. Therefore, we must differentiate between re-infection and persistent MG infection, and approach them accordingly. The genetic mechanisms of DNA variation in the MgPa operon and antibiotic resistance must be considered for the management of multicolored infection. In this respect, the unique genetic characteristics of MG will be described in detail. We hope that with this manuscript, clinicians can expand their understanding of recurrent MG infections and better choose an appropriate treatment for the infection in clinical setting.
REFERENCES
1.Delva D. Social implications of sexually transmitted diseases. Can Fam Physician. 1983. 29:1933–6.
2.Anderson RM. Transmission dynamics of sexually transmitted infections. Holmes KK, Sparling PF, Mardh PA, Lemon SM, Stamm WE, Piot P, editors. editors.Sexually transmitted diseases. 3rd ed.New York: McGraw-Hill;1999. p. 25–37.
3.Takahashi S., Hamasuna R., Yasuda M., Ishikawa K., Hayami H., Uehara S, et al. Nationwide surveillance of the antimicrobial susceptibility of Chlamydia trachomatis from male urethritis in Japan. J Infect Chemother. 2016. 22:581–6.
4.Krashin JW., Koumans EH., Bradshaw-Sydnor AC., Braxton JR., Evan Secor W., Sawyer MK, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010. 37:440–4.

5.Papp JR., Abrams AJ., Nash E., Katz AR., Kirkcaldy RD., OʼConnor NP, et al. Azithromycin resistance and decreased ceftriaxone susceptibility in Neisseria gonorrhoeae, Hawaii, USA. Emerg Infect Dis. 2017. 23:830–2.

6.Shipitsyna E., Rumyantseva T., Golparian D., Khayrullina G., Lagos AC., Edelstein I, et al. Prevalence of macrolide and fluoroquinolone resistance-mediating mutations in Mycoplasma genitalium in five cities in Russia and Estonia. PLoS One. 2017. 12:e0175763.

7.Manhart LE., Jensen JS., Bradshaw CS., Golden MR., Martin DH. Efficacy of antimicrobial therapy for mycoplasma genitalium infections. Clin Infect Dis. 2015. 61(Suppl 8):S802–17.
8.Jensen JS., Bradshaw C. Management of Mycoplasma genitalium infections-can we hit a moving target? BMC Infect Dis. 2015. 15:343.

9.Nijhuis RH., Severs TT., Van der Vegt DS., Van Zwet AA., Kusters JG. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015. 70:2515–8.
10.Salado-Rasmussen K., Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: a Danish nationwide retrospective survey. Clin Infect Dis. 2014. 59:24–30.

11.Horner P., Blee K., Adams E. Time to manage Mycoplasma genitalium as an STI: but not with azithromycin 1 g! Curr Opin Infect Dis. 2014. 27:68–74.
12.Deguchi T., Kikuchi M., Yasuda M., Ito S. Multidrug-resistant Mycoplasma genitalium is increasing. Clin Infect Dis. 2016. 62:405–6.
13.Serra-Pladevall J., Barbera MJ., Rodriguez S., Bartolome-Comas R., Roig G., Juve R, et al. Neisseria gonorrhoeae antimicrobial susceptibility in Barcelona: penA, ponA, mtrR, and porB mutations and NG-MAST sequence types associated with decreased susceptibility to cephalosporins. Eur J Clin Microbiol Infect Dis. 2016. 35:1549–56.

14.Hamasuna R. Mycoplasma genitalium in male urethritis: diagnosis and treatment in Japan. Int J Urol. 2013. 20:676–84.
15.Mondeja BA., Rodriguez NM., Barroto B., Blanco O., Jensen JS. Antimicrobial susceptibility patterns of recent Cuban Mycoplasma genitalium isolates determined by a modified cell-culture-based method. PLoS One. 2016. 11:e0162924.

16.Dupin N., Bijaoui G., Schwarzinger M., Ernault P., Gerhardt P., Jdid R, et al. Detection and quantification of Mycoplasma genitalium in male patients with urethritis. Clin Infect Dis. 2003. 37:602–5.

17.Falkow S., Isberg RR., Portnoy DA. The interaction of bacteria with mammalian cells. Annu Rev Cell Biol. 1992. 8:333–63.

18.Olive AJ., Sassetti CM. Metabolic crosstalk between host and pathogen: sensing, adapting and competing. Nat Rev Microbiol. 2016. 14:221–34.

19.Imming P., Sinning C., Meyer A. Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006. 5:821–34.

20.Briken V. Molecular mechanisms of host-pathogen interactions and their potential for the discovery of new drug targets. Curr Drug Targets. 2008. 9:150–7.

22.Koike S., Yoshitani S., Kobayashi Y., Tanaka K. Phylogenetic analysis of fiber-associated rumen bacterial community and PCR detection of uncultured bacteria. FEMS Microbiol Lett. 2003. 229:23–30.

23.Riley DE., Berger RE., Miner DC., Krieger JN. Diverse and related 16S rRNA-encoding DNA sequences in prostate tissues of men with chronic prostatitis. J Clin Microbiol. 1998. 36:1646–52.

24.Garza-Ramos G., Xiong L., Zhong P., Mankin A. Binding site of macrolide antibiotics on the ribosome: new resistance mutation identifies a specific interaction of ketolides with rRNA. J Bacteriol. 2001. 183:6898–907.

25.Drlica K., Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997. 61:377–92.

26.Blango MG., Mulvey MA. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob Agents Chemother. 2010. 54:1855–63.
27.Lee G., Romih R., Zupancic D. Cystitis: from urothelial cell biology to clinical applications. Biomed Res Int. 2014. DOI: doi: 10.1155/2014/473536.

28.NCBI. Taxonomy [Internet]. Bethesda: NCBI;2017. [cited 2017 Jun 10]. Available from:. https://www.ncbi.nlm.nih.gov/Taxonomy.
29.Taylor-Robinson D., Furr PM. Failure of Mycoplasma pneumoniae infection to confer protection against Mycoplasma genitalium: observations from a mouse model. J Med Microbiol. 2001. 50:383–4.

30.Wang RY., Grandinetti T., Shih JW., Weiss SH., Haley CL., Hayes MM, et al. Mycoplasma genitalium infection and host antibody immune response in patients infected by HIV, patients attending STD clinics and in healthy blood donors. FEMS Immunol Med Microbiol. 1997. 19:237–45.

31.Fraser CM., Gocayne JD., White O., Adams MD., Clayton RA., Fleischmann RD, et al. The minimal gene complement of Mycoplasma genitalium. Science. 1995. 270:397–403.
32.Razin S., Yogev D., Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998. 62:1094–156.

33.Ma L., Jensen JS., Myers L., Burnett J., Welch M., Jia Q, et al. Mycoplasma genitalium: an efficient strategy to generate genetic variation from a minimal genome. Mol Microbiol. 2007. 66:220–36.

35.Reinton N., Moi H., Olsen AO., Zarabyan N., Bjerner J., Tønseth TM, et al. Anatomic distribution of Neisseria gonorrhoeae, Chlamydia trachomatis and Mycoplasma genitalium infections in men who have sex with men. Sex Health. 2013. 10:199–203.

36.Baseman JB., Dallo SF., Tully JG., Rose DL. Isolation and characterization of Mycoplasma genitalium strains from the human respiratory tract. J Clin Microbiol. 1988. 26:2266–9.

37.Taylor-Robinson D., Jensen JS. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011. 24:498–514.

38.Mernaugh GR., Dallo SF., Holt SC., Baseman JB. Properties of adhering and nonadhering populations of Mycoplasma genitalium. Clin Infect Dis. 1993. 17(Suppl 1):S69–78.

39.Ma L., Jensen JS., Mancuso M., Hamasuna R., Jia Q., McGowin CL, et al. Variability of trinucleotide tandem repeats in the MgPa operon and its repetitive chromosomal elements in Mycoplasma genitalium. J Med Microbiol. 2012. 61:191–7.

40.Ma L., Jensen JS., Mancuso M., Hamasuna R., Jia Q., McGowin CL, et al. Genetic variation in the complete MgPa operon and its repetitive chromosomal elements in clinical strains of Mycoplasma genitalium. PLoS One. 2010. 5:e15660.

41.Iverson-Cabral SL., Astete SG., Cohen CR., Rocha EP., Totten PA. Intrastrain heterogeneity of the mgpB gene in Mycoplasma genitalium is extensive in vitro and in vivo and suggests that variation is generated via recombination with repetitive chromosomal sequences. Infect Immun. 2006. 74:3715–26.
42.Dallo SF., Baseman JB. Adhesin gene of Mycoplasma genitalium exists as multiple copies. Microb Pathog. 1991. 10:475–80.

43.Hu PC., Schaper U., Collier AM., Clyde WA Jr., Horikawa M., Huang YS, et al. A Mycoplasma genitalium protein resembling the Mycoplasma pneumoniae attachment protein. Infect Immun. 1987. 55:1126–31.

44.Clyde WA Jr., Hu PC. Antigenic determinants of the attachment protein of Mycoplasma pneumoniae shared by other pathogenic Mycoplasma species. Infect Immun. 1986. 51:690–2.

45.Burgos R., Pich OQ., Ferrer-Navarro M., Baseman JB., Querol E., Pinol J. Mycoplasma genitalium P140 and P110 cytadhesins are reciprocally stabilized and required for cell adhesion and terminal-organelle development. J Bacteriol. 2006. 188:8627–37.
46.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006. 124:783–801.

47.Schmid-Hempel P. Immune defence, parasite evasion strategies and their relevance for ‘macroscopic phenomenaʼ such as virulence. Philos Trans R Soc Lond B Biol Sci. 2009. 364:85–98.

48.Hornef MW., Wick MJ., Rhen M., Normark S. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat Immunol. 2002. 3:1033–40.

49.NCBI. Nucleotide [Internet]. Bethesda: NCBI;2017. [cited 2017 Jun 10]. Available from:. https://www.ncbi.nlm.nih.gov/nuccore/.
50.Ma L., Mancuso M., Williams JA., Van Der Pol B., Fortenberry JD., Jia Q, et al. Extensive variation and rapid shift of the MG192 sequence in Mycoplasma genitalium strains from patients with chronic infection. Infect Immun. 2014. 82:1326–34.

51.Ma L., Jensen JS., Mancuso M., Myers L., Martin DH. Kinetics of genetic variation of the Mycoplasma genitalium MG192 gene in experimentally infected chimpanzees. Infect Immun. 2015. 84:747–53.

52.Taylor-Robinson D. The Harrison lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995. 71:1–8.

53.Jensen JS. Mycoplasma genitalium: the aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004. 18:1–11.

54.Jensen JS., Cusini M., Gomberg M., Moi H. 2016 European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2016. 30:1650–6.

55.Deguchi T., Ito S., Yasuda M., Kondo H., Yamada Y., Nakane K, et al. Emergence of Mycoplasma genitalium with clinically significant fluoroquinolone resistance conferred by amino acid changes both in GyrA and ParC in Japan. J Infect Chemother. 2017. 23:648–50.

56.Braam JF., van Dommelen L., Henquet CJ., van de Bovenkamp JH., Kusters JG. Multidrug-resistant Mycoplasma genitalium infections in Europe. Eur J Clin Microbiol Infect Dis. 2017 Mar 30. [Epub].DOI: doi: 10.1007/s10096-017-2969-9.

57.Murray GL., Bradshaw CS., Bissessor M., Danielewski J., Garland SM., Jensen JS, et al. Increasing macrolide and fluoroquinolone resistance in mycoplasma genitalium. Emerg Infect Dis. 2017. 23:809–12.

58.Hamasuna R., Osada Y., Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with Vero cells. J Clin Microbiol. 2007. 45:847–50.
59.Bocchetta M., Xiong L., Mankin AS. 23S rRNA positions essential for tRNA binding in ribosomal functional sites. Proc Natl Acad Sci U S A. 1998. 95:3525–30.

60.Beringer M., Rodnina MV. Importance of tRNA interactions with 23S rRNA for peptide bond formation on the ribosome: studies with substrate analogs. Biol Chem. 2007. 388:687–91.

61.Brosius J., Dull TJ., Noller HF. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980. 77:201–4.

62.Harrod R., Lovett PS. Peptide inhibitors of peptidyltransferase alter the conformation of domains IV and V of large subunit rRNA: a model for nascent peptide control of translation. Proc Natl Acad Sci U S A. 1995. 92:8650–4.

63.Schlunzen F., Zarivach R., Harms J., Bashan A., Tocilj A., Albrecht R, et al. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature. 2001. 413:814–21.

64.Hansen JL., Ippolito JA., Ban N., Nissen P., Moore PB., Steitz TA. The structures of four macrolide antibiotics bound to the large ribosomal subunit. Mol Cell. 2002. 10:117–28.

65.Kim JH., Kim JY., Yoo CH., Seo WH., Yoo Y., Song DJ, et al. Macrolide resistance and its impacts on M. Pneumoniae pneumonia in children: comparison of two recent epidemics in Korea. Allergy Asthma Immunol Res. 2017. 9:340–6.

66.Anagrius C., Lore B., Jensen JS. Treatment of Mycoplasma genitalium. Observations from a Swedish STD clinic. PLoS One. 2013. 8:e61481.

67.Couldwell DL., Lewis DA. Mycoplasma genitalium infection: current treatment options, therapeutic failure, and resistance-associated mutations. Infect Drug Resist. 2015. 8:147–61.
68.Couldwell DL., Tagg KA., Jeoffreys NJ., Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013. 24:822–8.

69.Kikuchi M., Ito S., Yasuda M., Tsuchiya T., Hatazaki K., Takanashi M, et al. Remarkable increase in fluoroquinolone-resistant Mycoplasma genitalium in Japan. J Antimicrob Chemother. 2014. 69:2376–82.

70.Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol. 2002. 3:430–40.

71.Takahashi S., Hamasuna R., Yasuda M., Ito S., Ito K., Kawai S, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013. 19:941–5.
Fig 1.
Phylogenetic tree of 16S rRNA gene sequences reveals that Mycoplasma pneumoniae M129 is closely related with Mycoplasma genitalium (MG). NR074611.1 is MG strain G37 (16S ribosomal RNA), NC000912.1 is M. pneumoniae M129 complete genome (16S ribosomal RNA), NC010503.1 is Ureaplasma parvum serotype 3 str. ATCC27815, complete genome (16S ribosomal RNA), NC011374 is Ureaplasma urealyticum serotype 10 str. ATCC33699, complete genome (16S ribosomal RNA), and AB680681.1 is Mycoplasma hominis gene for 16S ribosomal RNA, strain NBRC14850.

Fig. 2.
Schematic view of Mycoplasma genitalium (MG). It appears as a flask or pear-shaped cell with a narrow terminal structure (A). This structure is crucial for MG to attach onto the host cell surfaces.
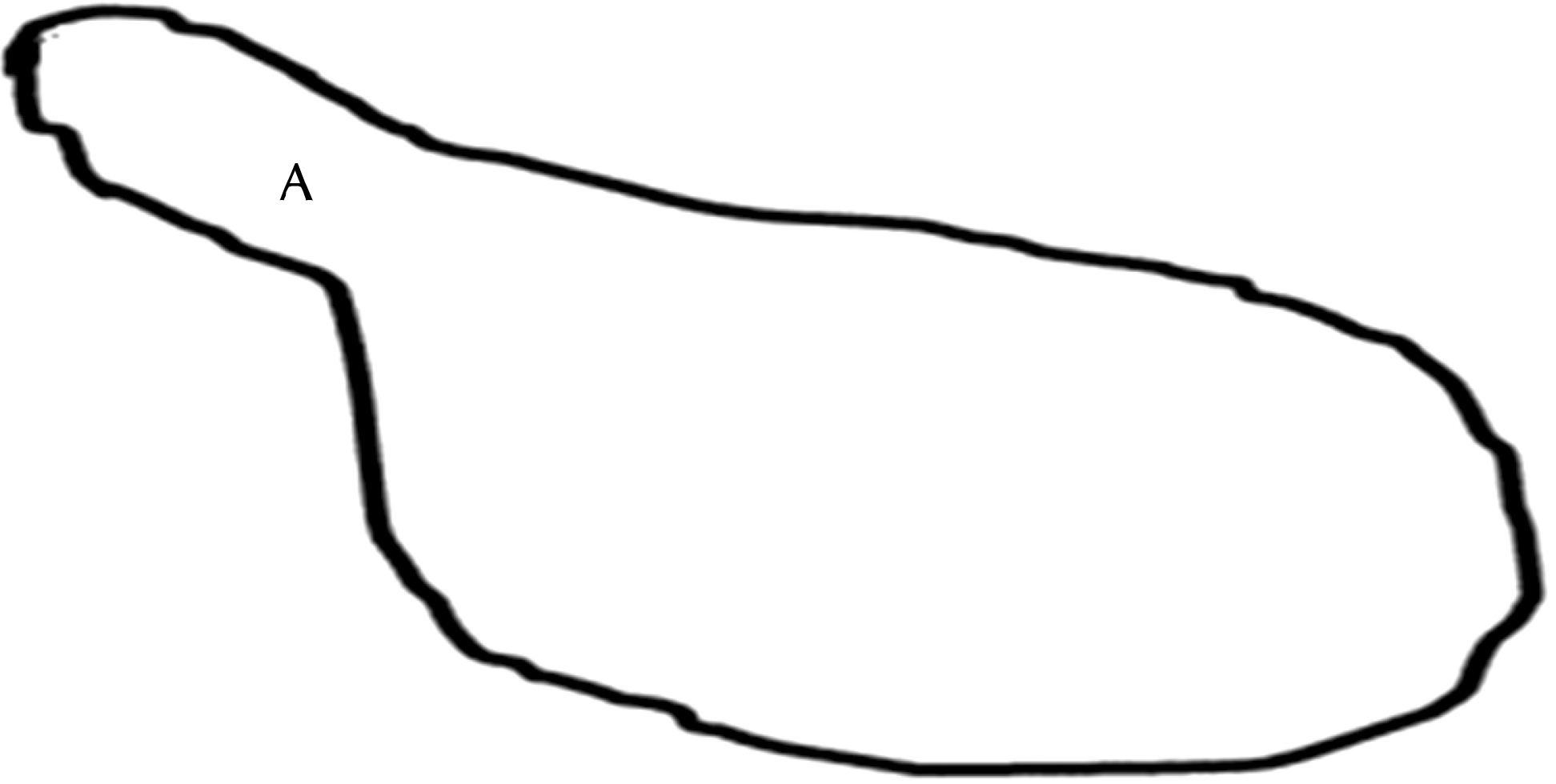
Fig. 3.
Complete genome sequence in MgPa and MgPar genes of the Mycoplasma genitalium (MG) G37 strain. The expression sites for each of the three genes are present in only one copy; but there are nine repetitive elements in the form of truncated copies of MG191 and MG192 genes dispersed throughout the genome, designated as MgPa repeats or MgPar sequences [31,39,40].
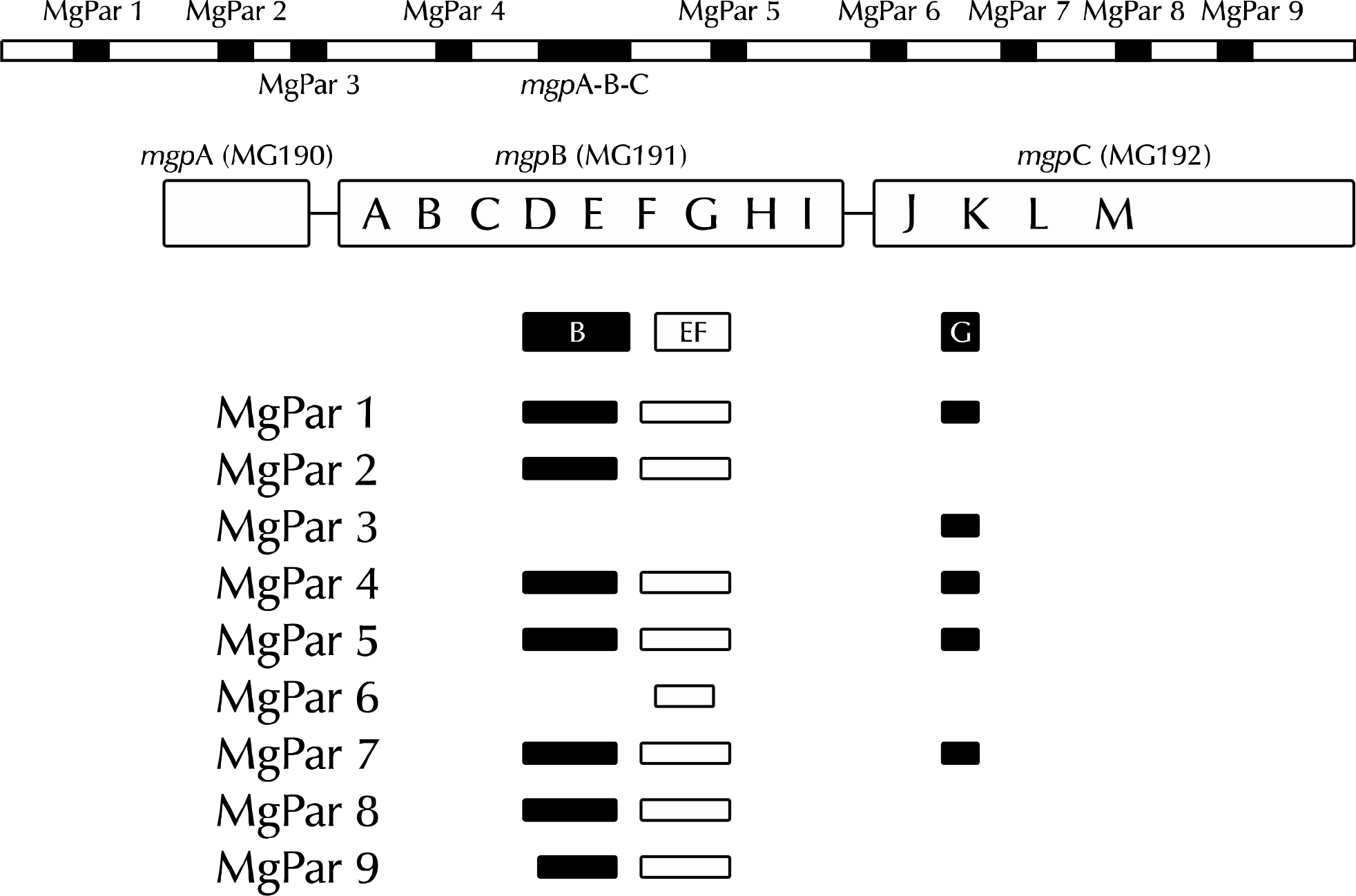
Fig. 4.
Twenty mgpB DNA sequences from the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/nuccore/) are evaluated for their homology using the Clustal W alignment algorithm (http://www.genome.jp/tools/clustalw/). Locations in the MG191 gene are indicated in the upper area. There are three areas of hot genetic variety in the mgpB gene (520-1,040 bp for B area, 2,340-2,990 bp for EF area, 3,250-3,640 bp for G area). The gray colors represent matching, while the black colors represent mismatching (http://multalin.toulouse.inra.fr/). MG: Mycoplasma genitalium.
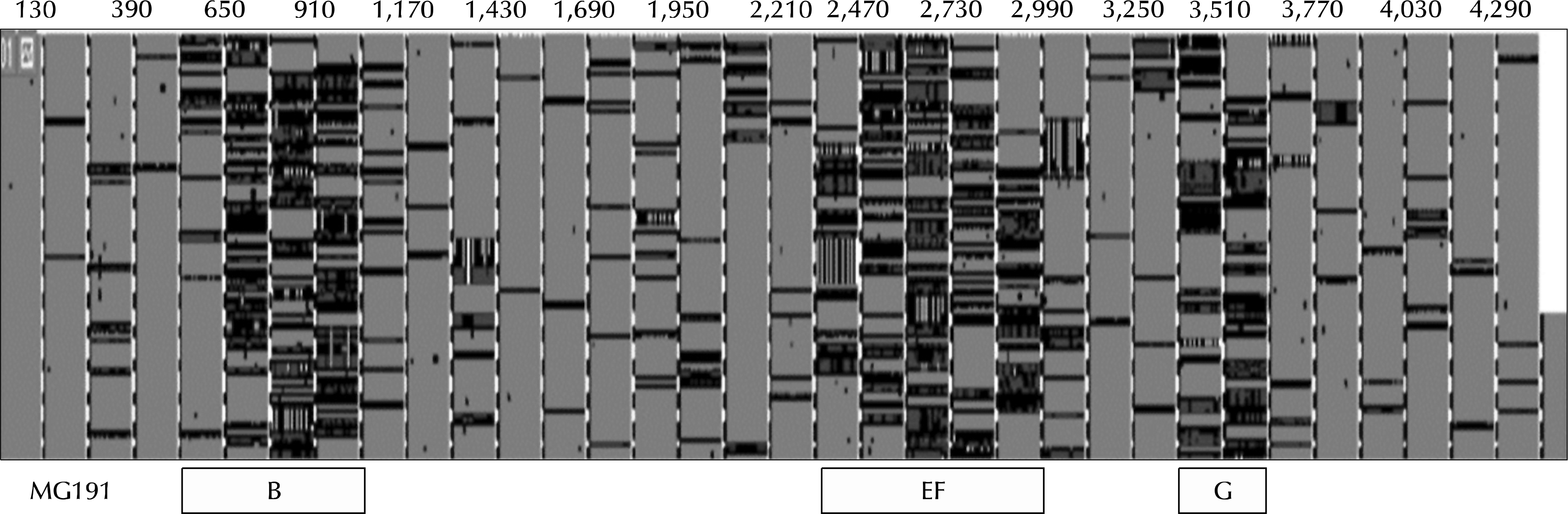
Fig. 5.
Sixty-four partial mgpC DNA sequences (Supplementary Table 2) from the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/nuccore/) are collected and evaluated for their homology using the Clustal W alignment algorithm (http://www.genome.jp/tools/clustalw/). Locations in the mgpB gene are indicated in the upper area. The gray colors represent matching, while the black colors represent mismatching (http://multalin.toulouse.inra.fr).

Fig. 6.
Twenty-four partial mgpC DNA sequences (Supplementary Table 3) from patient no. 126 are evaluated from the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/nuccore/) to investigate the homology using the Clustal W alignment algorithm (http://www.genome.jp/ tools/clustalw/). Ma et al. [50] serially examined the DNA sequence changes in the mgpC gene in serial culturing periods (from the starting point to 4th subculturing period). The DNA sequences of MG192 changed rapidly in vitro within a short period of time. The gray colors represent matching, while the black colors represent mismatching (http://multalin.toulouse.inra.fr/multalin/multalin.html). MG: Mycoplasma genitalium.

Fig. 7.
Phylogenetic analysis of the MG192 variable sequences from serially cultured in vitro samples from MG infected patients no. 61 and no. 126. Time intervals are described in Table 4. There are greater variations in the MG192 gene during sequential cultures, but the variances are more closely related within an individual patient than between the patients. MG: Mycoplasma genitalium.
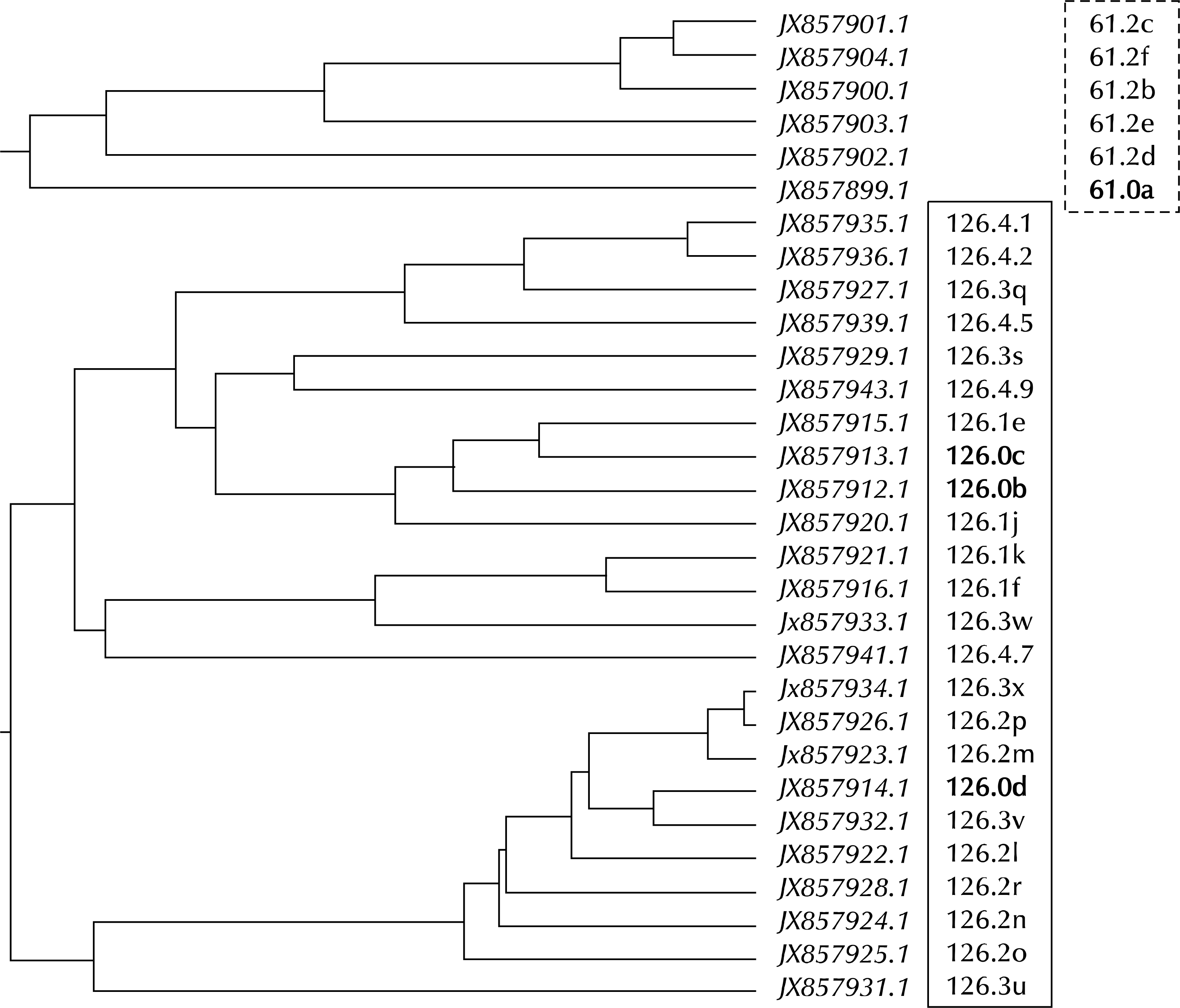
Fig. 8.
Ma et al. [51] intraurethrally inoculated a single cloned Mycoplasma genitalium (MG) strain G37 into one male chimpanzee's urethra. Urethral swabs were obtained at different time points (5 weeks, 9 weeks, and 11 weeks). Phylogenetic analysis in the MG192 gene revealed several MG192 sequences variants during an in vivo study.
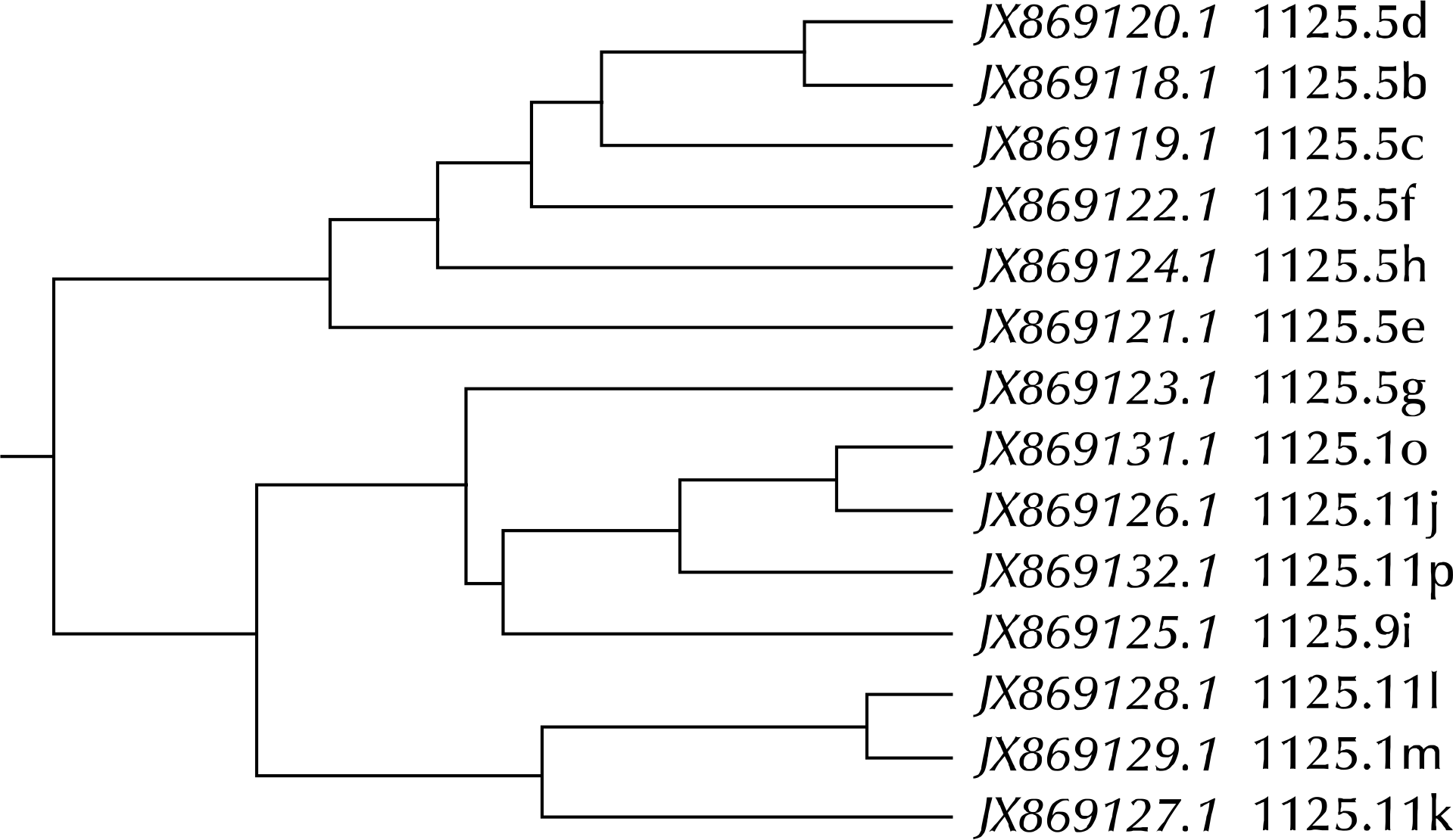
Fig. 9.
JX857899.1(61.0a) is the partial DNA sequences of MG192 gene at the starting point (11/20/2000) obtained from patient no. 61, as presented in Fig. 7. The JX857903.1(61.2e) is the partial DNA sequences of MG192 gene obtained from the same patient post 6 months (05/07/2001). EF117289.1 is the partial DNA sequences of MG strain G37 MgPar 8 region genomic sequence, FJ872575.1 is the partial DNA sequences of MG strain M2321 MgPar 2 region genomic sequence, and FJ872582.1 is the partial DNA sequences of MG strain M2321 MgPar 9 region genomic sequence. JX857903.1(61.2e) may get some AGT repeats in the original MG192 gene (JX857899.1), and newly mutated isolate (JX857903.1) is closely related to the partial DNA sequences of MG strain G37 MgPar 8 region genomic sequence (EF117289.1) (dotted box). MG: Mycoplasma genitalium.
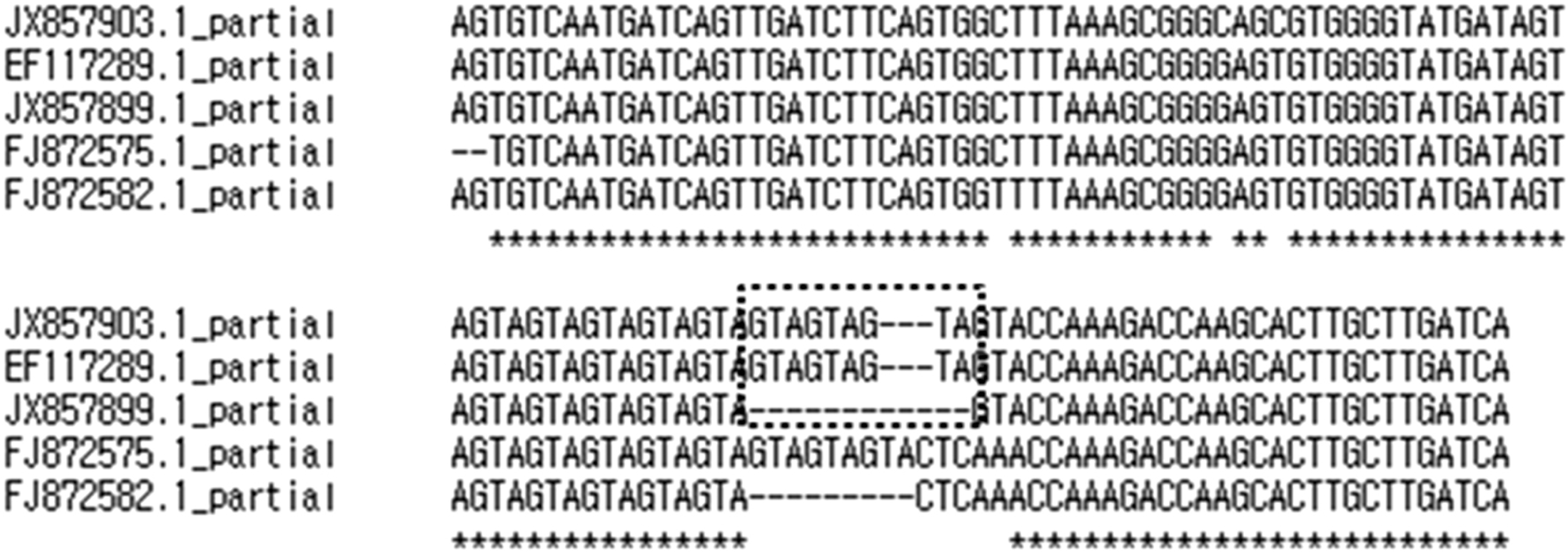
Fig. 10.
A schematic view of homologous recombination between MG192 and MgPar 8 in the MG type strain G37 during in vitro passage. A presents the alignment of a portion of the MG192 variable region and MgPar 8 in G37-P1, while B demonstrates the alignment of a portion of the recombined MG192 variable region and MgPar 8 in G37-P35, clearly depicting the sequence exchange at the recombination sites. Arrows indicate where sequence exchanges took place, with the reciprocal exchange (gene cross-over) shown by two arrows (area II) and gene conversion shown by one arrow (area I). MG: Mycoplasma genitalium.
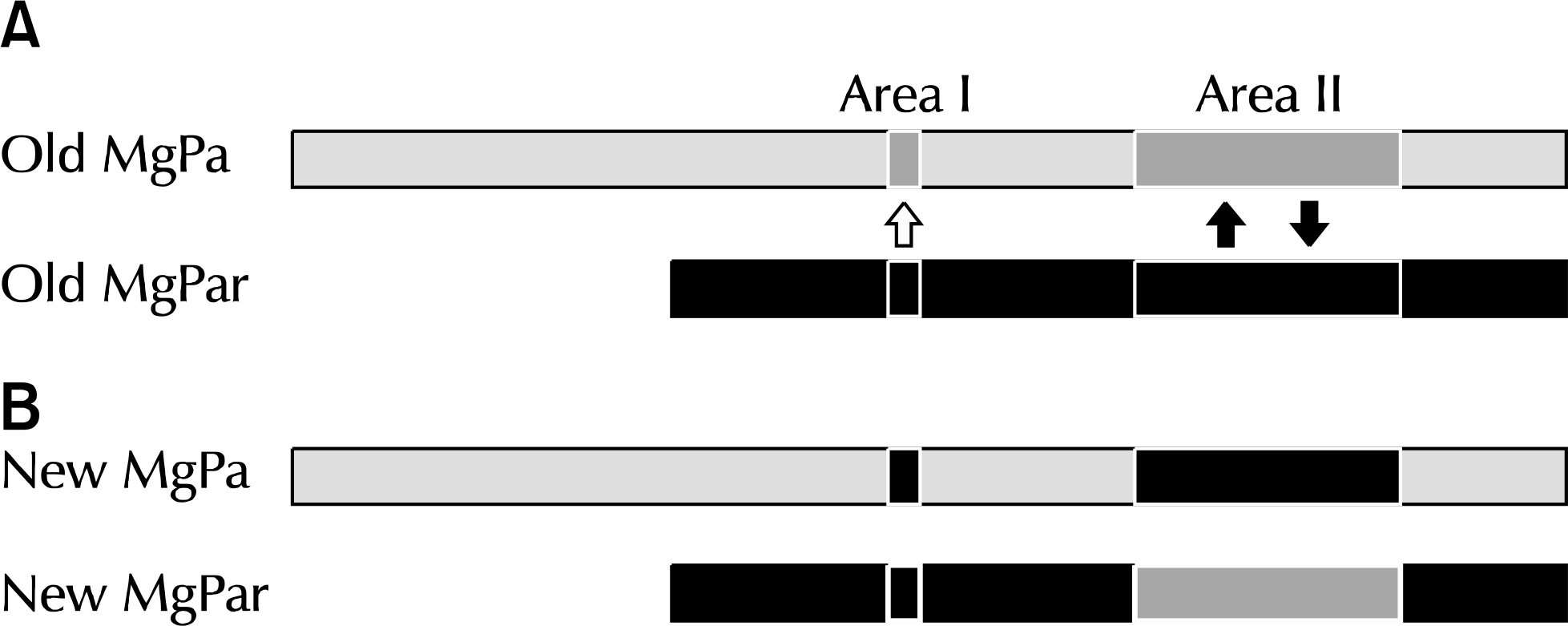
Table 1.
Taxonomical classification of Mycoplasmataceae family
Table 2.
Structural characteristics of Mycoplasma genitalium
Table 3.
Tissue tropisms of Mycoplasmas
| Species | Primary site of colonization | Pathogenicity | |
|---|---|---|---|
| Oropharynx | Urogenital tract | ||
| Mycoplasma hominis | Y | Y | Y |
| Ureaplasma urealyticum | Y | Y | Y |
| Mycoplasma pneumonia | Y | N | Y |
| Mycoplasma genitalium | ?Y | Y | Y |
Table 4.
Sequential cultures of Mycoplasma genitalium (MG) from two infected parents and unique MG192 variants
| Patient no. | Date | Clonesʼ no. | Unique MG192 sequences variant(s) |
|---|---|---|---|
| 61 | 11/20/2000 | 8 | 61.0aa) |
| 5/7/2001 | 6 | 61.2b, 61.2c, 61.2d, 61.2e, 61.2f | |
| 126 | 10/23/2001 | 10 | 126.0a, 126.0b, 126.0c, 126.0da) |
| 1/15/2002 | 10 | 126.1e, 126.1f, 126.1g, 126.1h, 126.1i, 126.1j, 126.1k | |
| 6/26/2002 | 10 | 126.2l, 126.2m, 126.2n, 126.2o, 126.2p | |
| 9/17/2002 | 10 | 126.3q, 126.3r, 126.3s, 126.3t, 126.3u, 126.3v, 126.3w, 126.3x | |
| 12/3/2002 | 10 | 126.4.1, 126.4.2, 126.4.3, 126.4.4, 126.4.5, 126.4.6, 126.4.7, 126.4.8, 126.4.9 |
Table 5.
Topoisomerase IIA family in Mycoplasma genitalium
| Enzyme | Type | Structure | Genes | Annotations | Locationa) | Gene name |
|---|---|---|---|---|---|---|
| Gyrase | Topoisomerase IIA | Heterodimer | gyrA | DNA gyrase subunit A | 4812..7322 | AFQ04307.1 |
| (AB/AB) | gyrB | DNA gyrase subunit B | 2845..4797 | AFQ04306.1 | ||
| Topo IV | Topoisomerase IIA | Heterodimer | ParC | DNA topoisomerase IV, A subunit | 242059..244404 | AFQ04524.1 |
| (AB/AB) | ParE | DNA topoisomerase IV, B subunit | 240158..242059 | AFQ04523.1 |
Table 6.
List of common DNA mutations in toposiomerase IIA genes that change their amino acid sequences, which may inactivate moxifloxacin treatment in Mycoplasma genitalium infection [67-69]
Supplementary Table 1.
Mycoplasma genitalium mgpB sequences from the National Center for Biotechnology Information
Supplementary Table 2.
Mycoplasma genitalium mgpC sequences from the National Center for Biotechnology Information
Supplementary Table 3.
Mycoplasma genitalium mgpC sequences from patient No. 126 from the National Center for Biotechnology Information
Supplementary Table 4.
Mycoplasma genitalium mgpC sequences from patient No. 1125 from the National Center for Biotechnology Information




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download