Abstract
To date, there has not been an establishment of guidelines for urinary tract infections, due to limited domestic data in Korea, unlike other North American and European countries. The clinical characteristics, etiology, and antimicrobial susceptibility of urinary tract infections vary from country to country. Moreover, despite the same disease, antibiotic necessary to treat it may vary from country to country. Therefore, it is necessary to establish a guideline that is relevant to a specific country. However, in Korea, domestic data have been limited, and thus, guidelines considering the epidemiological characteristics pertaining specifically to Korea do not exist. Herein, describe a guideline that was developed by the committee of The Korean Association of Urogenital Tract Infection and Inflammation, which covers only the uncomplicated urinary tract infections, as covering all parts in the first production is difficult.
Go to : 
REFERENCES
1.Stamm WE., Hooton TM. Management of urinary tract infections in adults. N Engl J Med. 1993. 329:1328–34.

2.Bradbury SM. Collection of urine specimens in general practice: to clean or not to clean? J R Coll Gen Pract. 1988. 38:363–5.
3.Lifshitz E., Kramer L. Outpatient urine culture: does collection technique matter? Arch Intern Med. 2000. 160:2537–40.
4.Foxman B., Brown P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am. 2003. 17:227–41.
5.Fihn SD. Clinical practice. Acute uncomplicated urinary tract infection in women. N Engl J Med. 2003. 349:259–66.
6.Gupta K., Hooton TM., Naber KG., Wullt B., Colgan R., Miller LG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011. 52:e103–20.

7.Kim ME., Ha US., Cho YH. Prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in female outpatients in South Korea: a multicentre study in 2006. Int J Antimicrob Agents. 2008. 31(Suppl 1):S15–8.

8.Lee SJ., Lee DS., Choe HS., Shim BS., Kim CS., Kim ME, et al. Antimicrobial resistance in community-acquired urinary tract infections: results from the Korean Antimicrobial Resistance Monitoring System. J Infect Chemother. 2011. 17:440–6.
9.Rafalsky V., Andreeva I., Rjabkova E. Quinolones for uncomplicated acute cystitis in women. Cochrane Database Syst Rev. 2006. 3:CD003597.

10.Nicolle LE. Pivmecillinam in the treatment of urinary tract infections. J Antimicrob Chemother. 2000. 46(Suppl 1):35–9. discussion 63-5.

11.Gupta K., Hooton TM., Roberts PL., Stamm WE. Short-course nitrofurantoin for the treatment of acute uncomplicated cystitis in women. Arch Intern Med. 2007. 167:2207–12.

12.Warren JW., Abrutyn E., Hebel JR., Johnson JR., Schaeffer AJ., Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA) Clin Infect Dis. 1999. 29:745–58.

13.Gupta K., Stamm WE. Outcomes associated with trimethoprim/sulphamethoxazole (TMP/SMX) therapy in TMP/SMX resistant community-acquired UTI. Int J Antimicrob Agents. 2002. 19:554–6.

14.Nicolle LE., Bradley S., Colgan R., Rice JC., Schaeffer A., Hooton TM. Infectious Diseases Society of America; American Society of Nephrology; American Geriatric Society. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005. 40:643–54.

15.Karlowsky JA., Hoban DJ., Decorby MR., Laing NM., Zhanel GG. Fluoroquinolone-resistant urinary isolates of Escherichia coli from outpatients are frequently multidrug resistant: results from the North American Urinary Tract Infection Collaborative Alliance-Quinolone Resistance study. Antimicrob Agents Chemother. 2006. 50:2251–4.
16.Zhanel GG., Hisanaga TL., Laing NM., DeCorby MR., Nichol KA., Weshnoweski B, et al. Antibiotic resistance in Escherichia coli outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents. 2006. 27:468–75.

17.Wie SH., Chang UI., Kim HW., Kim YS., Kim SY., Hur J, et al. Clinical features and antimicrobial resistance among clinical isolates of women with community-acquired acute pyelonephritis in 2001-2006. Infect Chemother. 2007. 39:9–16.
18.Ki M., Park T., Choi B., Foxman B. The epidemiology of acute pyelonephritis in South Korea, 1997-1999. Am J Epidemiol. 2004. 160:985–93.

19.Lee DG., Jeon SH., Lee CH., Lee SJ., Kim JI., Chang SG. Acute pyelonephritis: clinical characteristics and the role of the surgical treatment. J Korean Med Sci. 2009. 24:296–301.

20.Lee DS., Choe HS., Lee SJ., Bae WJ., Cho HJ., Yoon BI, et al. Antimicrobial susceptibility pattern and epidemiology of female urinary tract infections in South Korea, 2010-2011. Antimicrob Agents Chemother. 2013. 57:5384–93.

21.Scholes D., Hooton TM., Roberts PL., Gupta K., Stapleton AE., Stamm WE. Risk factors associated with acute pyelonephritis in healthy women. Ann Intern Med. 2005. 142:20–7.

22.Busch R., Huland H. Correlation of symptoms and results of direct bacterial localization in patients with urinary tract infections. J Urol. 1984. 132:282–5.
23.King C., Garcia Alvarez L., Holmes A., Moore L., Galletly T., Aylin P. Risk factors for healthcare-associated urinary tract infection and their applications in surveillance using hospital administrative data: a systematic review. J Hosp Infect. 2012. 82:219–26.

24.Jung YH., Cho IR., Lee SE., Lee KC., Kim JG., Jeon JS, et al. Comparative analysis of clinical parameters in acute pyelonephritis. Korean J Urol. 2007. 48:29–34.

25.Hwang BY., Lee JG., Park DW., Lee YJ., Kim SB., Eom JS, et al. Antimicrobial susceptibility of causative microorganisms in adults with acute pyelonephritis at one university-affiliated hospital in southwestern Seoul. Infect Chemother. 2003. 35:277–82.
26.Wie SH., Choi SM., Lee DG., Kim SY., Kim SI., Yoo JH, et al. Antibiotic sensitivity of the causative organisms and use of antibiotics in women with community-acquired acute pyelonephritis. Korean J Infect Dis. 2002. 34:353–9.
27.Park JH., Wee JH., Choi SP., Park KN. Serum procalcitonin level for the prediction of severity in women with acute pyelonephritis in the ED: value of procalcitonin in acute pyelonephritis. Am J Emerg Med. 2013. 31:1092–7.

28.Ha YE., Kang CI., Wi YM., Chung DR., Kang ES., Lee NY, et al. Diagnostic usefulness of procalcitonin as a marker of bacteremia in patients with acute pyelonephritis. Scand J Clin Lab Invest. 2013. 73:444–8.

29.Velasco M., Martinez JA., Moreno-Martinez A., Horcajada JP., Ruiz J., Barranco M, et al. Blood cultures for women with uncomplicated acute pyelonephritis: are they necessary? Clin Infect Dis. 2003. 37:1127–30.

30.Mitterberger M., Pinggera GM., Colleselli D., Bartsch G., Strasser H., Steppan I, et al. Acute pyelonephritis: comparison of diagnosis with computed tomography and contrast-enhanced ultrasonography. BJU Int. 2008. 101:341–4.

31.Browne RF., Zwirewich C., Torreggiani WC. Imaging of urinary tract infection in the adult. Eur Radiol. 2004. 14(Suppl 3):E168–83.

32.Paick SH., Choo GY., Baek M., Bae SR., Kim HG., Lho YS, et al. Clinical value of acute pyelonephritis grade based on computed tomography in predicting severity and course of acute pyelonephritis. J Comput Assist Tomogr. 2013. 37:440–2.

33.Ha SK., Seo JK., Kim SJ., Park SH., Park CH., Lee HY, et al. Acute pyelonephritis focusing on perfusion defects on contrast enhanced computerized tomography(CT) scans and its clinical outcome. Korean J Intern Med. 1997. 12:122–7.
34.Naber KG., Schito G., Botto H., Palou J., Mazzei T. Surveillance study in Europe and Brazil on clinical aspects and Antimicrobial Resistance Epidemiology in Females with Cystitis (ARESC): implications for empiric therapy. Eur Urol. 2008. 54:1164–75.

35.Talan DA., Stamm WE., Hooton TM., Moran GJ., Burke T., Iravani A, et al. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis pyelonephritis in women: a randomized trial. JAMA. 2000. 283:1583–90.
36.Klausner HA., Brown P., Peterson J., Kaul S., Khashab M., Fisher AC, et al. A trial of levofloxacin 750 mg once daily for 5 days versus ciprofloxacin 400 mg and/or 500 mg twice daily for 10 days in the treatment of acute pyelonephritis. Curr Med Res Opin. 2007. 23:2637–45.
37.Peterson J., Kaul S., Khashab M., Fisher AC., Kahn JB. A double-blind, randomized comparison of levofloxacin 750 mg once-daily for five days with ciprofloxacin 400/500 mg twice-daily for 10 days for the treatment of complicated urinary tract infections and acute pyelonephritis. Urology. 2008. 71:17–22.

38.Cronberg S., Banke S., Bergman B., Boman H., Eilard T., Elbel E, et al. Fewer bacterial relapses after oral treatment with norfloxacin than with ceftibuten in acute pyelonephritis initially treated with intravenous cefuroxime. Scand J Infect Dis. 2001. 33:339–43.
39.Colgan R., Williams M. Diagnosis and treatment of acute uncomplicated cystitis. Am Fam Physician. 2011. 84:771–6.
40.Stamm WE., McKevitt M., Counts GW. Acute renal infection in women: treatment with trimethoprim-sulfamethoxazole or ampicillin for two or six weeks. A randomized trial. Ann Intern Med. 1987. 106:341–5.
41.Richard GA., Klimberg IN., Fowler CL., Callery-D'Amico S., Kim SS. Levofloxacin versus ciprofloxacin versus lomefloxacin in acute pyelonephritis. Urology. 1998. 52:51–5.

42.Rubin RH., Shapiro ED., Andriole VT., Davis RJ., Stamm WE. Evaluation of new anti-infective drugs for the treatment of urinary tract infection. Clin Infect Dis. 1992. 15(Suppl 1):S216–27.

43.Wells WG., Woods GL., Jiang Q., Gesser RM. Treatment of complicated urinary tract infection in adults: combined analysis of two randomized, double-blind, multicentre trials comparing ertapenem and ceftriaxone followed by appropriate oral therapy. J Antimicrob Chemother. 2004. 53(Suppl 2):ii67–74.

44.Mouton YJ., Beuscart C. Empirical monotherapy with meropenem in serious bacterial infections. Meropenem Study Group. J Antimicrob Chemother. 1995. 36(Suppl A):145–56.
45.Giamarellou H. Low-dosage cefepime as treatment for serious bacterial infections. J Antimicrob Chemother. 1993. 32(Suppl B):123–32.

46.Naber KG., Savov O., Salmen HC. Piperacillin 2 g/tazobactam 0.5 g is as effective as imipenem 0.5 g/cilastatin 0.5 g for the treatment of acute uncomplicated pyelonephritis and complicated urinary tract infections. Int J Antimicrob Agents. 2002. 19:95–103.

47.Naber KG., Llorens L., Kaniga K., Kotey P., Hedrich D., Redman R. Intravenous doripenem at 500 milligrams versus levofloxacin at 250 milligrams, with an option to switch to oral therapy, for treatment of complicated lower urinary tract infection and pyelonephritis. Antimicrob Agents Chemother. 2009. 53:3782–92.

Go to : 
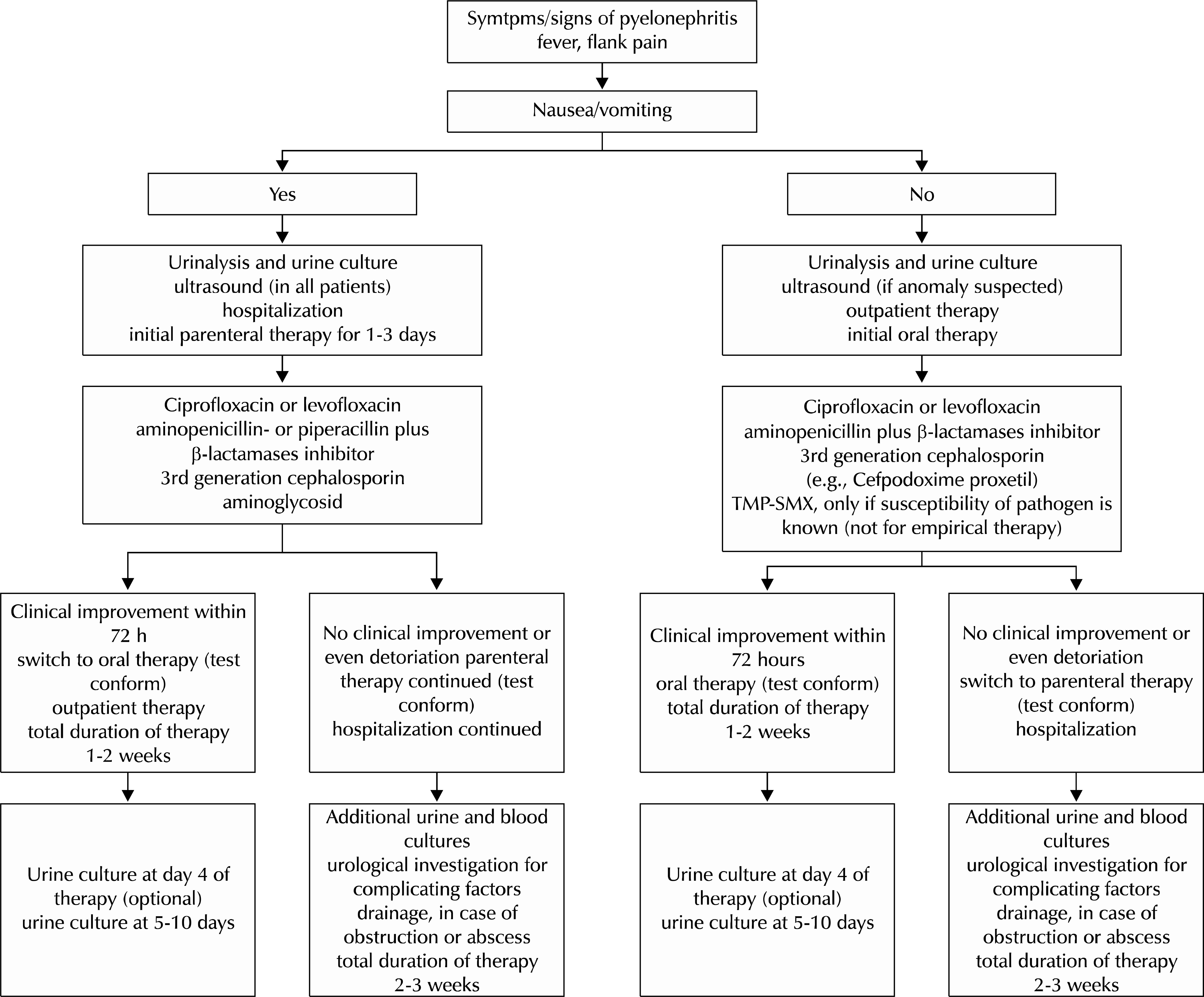 | Fig. 1.Algorithm for clinical treatment of acute uncomplicated pyelonephritis. TMP-SMX: trimethoprim-sulfamethoxazole. |
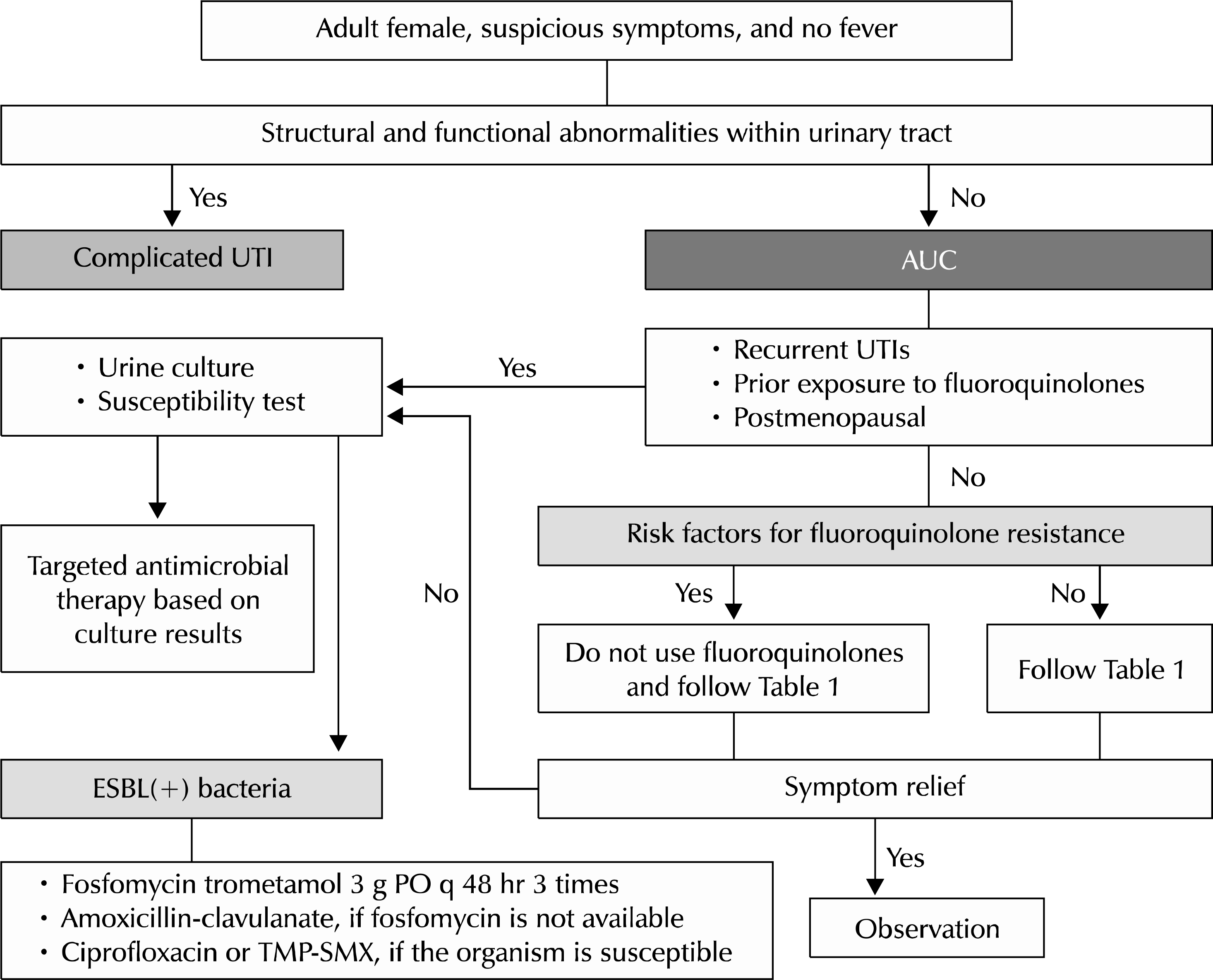 | Fig. 2.Algorithm for clinical management of acute uncomplicated cystitis (AUC). UTI: urinary tract infection, ESBL: extended spectrum beta-lactamase, TMP-SMX: trimethoprim-sulfamethoxazole. |
Table 1.
Empirical antibiotic therapy for acute uncomplicated cystitis
| Antibiotic | Daily dose (oral) | Duration of therapy (d) |
|---|---|---|
| Fosfomycin trometamol | 3 g qd | 1 |
| Pivmecillinama) | 400 mg tid | 3 |
| Nitrofurantoin macrocrystala) | 100 mg bid | 5-7 |
| -Lactams | ||
| Amoxicillin-clavulanic acid | 250/125 mg tid | 7 |
| 500/125 mg bid | ||
| Cefaclor | 250 mg tid | 7 |
| Cefdinir | 100 mg tid | 5-7 |
| Cefcapene pivoxil | 100 mg tid | 5-7 |
| Cefpodoxime prexetil | 100 mg bid | 5-7 |
| Fluoroquinolones | ||
| Ciprofloxacinb) | 500 mg bid | 3 |
| 500 mg SR qd | ||
| Tosufloxacinb) | 150 mg bid | 3 |
Table 2.
Factors that suggest a potential acute complicated pyelonephritis [23]
Table 3.
Initial therapy recommended for severe acute uncomplicated pyelonephritis
Table 4.
Initial empirical antimicrobial therapy in acute uncomplicated pyelonephritis in premenopausal women (oral therapy: mild and moderate acute uncomplicated pyelonephritis)
| Antibiotics | Daily dose | Duration of therapy (d) | Reference |
|---|---|---|---|
| Ciprofloxacina) | 500-750 mg bid | 7-10 | Talan et al. [35] |
| Levofloxacina) | 250-500 mg qd | 7-10 | Richard et al. [41] |
| Levofloxacin | 750 mg qd | 5 | Klausner et al. [36] |
| Peterson et al. [37] | |||
| Alternative (clinically equivalent to fluoroquinolone but not equivalent to bacteriological effect) | |||
| Cefpodoxime proxetil | 200 mg bid | 10 | Cronberg et al. [38] |
| Ceftibuten | 400 mg bid | 10 | Peterson et al. [37] |
| If an antibiotic susceptibility test is performed (not appropriate for primary treatment) | |||
| Trimethoprim-sulphamethoxazole | 160/800 mg bid | 14 | Rubin et al. [42] |
| Co-amoxiclavb),c) | 0.5/0.125 g tid | 14 | |
Table 5.
Antibiotic susceptibility (%)
Table 6.
Initial empirical antimicrobial therapy in acute uncomplicated pyelonephritis in premenopausal women (parenteral therapy: severe acute uncomplicated pyelonephritis)
| Antibiotic | Daily dose | Reference |
|---|---|---|
| Ciprofloxacin | 400 mg bid | Talan et al. [35] |
| Levofloxacina) | 250-500 mg qd | Richard et al. [41] |
| Levofloxacin | 750 mg qd | Klausner et al. [36] |
| Alternative | ||
| Cefotaximeb) | 2 g tid | |
| Ceftriaxonea),d) | 1-2 g qd | Wells et al. [43] |
| Ceftazidimeb) | 1-2 g tid | Mouton and Beuscart [44] |
| Cefepimea),d) | 1-2 g bid | Giamarellou [45] |
| Co-amoxiclavb),c) | 1.5 g tid | |
| Piperacillin/ | 2.5-4.5 g tid | Naber et al. [46] |
| tazobactama),d) | ||
| Gentamicinb) | 5 mg/kg qd | |
| Amikacinb) | 15 mg/kg qd | |
| Ertapenemd) | 1 g qd | Wells et al. [43] |
| Imipenem/cilastatind) | 0.5/0.5 g tid | Naber et al. [46] |
| Meropenemd) | 1 g tid | Mouton and Beuscart [44] |
| Doripenemd) | 0.5 g tid | Naber et al. [47] |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download