Abstract
Purpose:
Bacillus Calmette-Guerin (BCG) vaccination has been administered to most infants at birth in Korea; however, tuberculosis (TB) remains extant. TB can leave sequelae on chest radiography according to the immune response of the host. We investigated the symptoms of cystitis after intravesica instillations in bladder cancer, depending on the TB sequelae on chest radiography.
Materials and Methods:
One hundred forty-two patients with nonmuscle invasive bladder cancer (NMIBC) underwent transurethral resection and intravesical BCG therapy for bladder cancer. Patients received a BCG induction course―with or without a maintenance course―and were divided into the two groups: Group A, which included patients with visible sequelae of TB on chest radiography (n=31) and group B, which included patients without visible sequelae of TB (n=111). Cystitis symptoms of BCG intravesical therapy were compared between the two groups. The recurrence and progression rates of bladder cancer were also analyzed.
Results:
The overall rate of cystitis symptoms was 32.3% (10/31) in group A and 33.3% (37/111) in group B. One patient in group A and three in group B did not complete the treatment course due to severe cystitis symptoms (p=0.876). Pyuria was reported when cystitis symptoms occurred in 80% (8/10) in group A and 56.8% (21/37) in group B. The recurrence and progression rates were not different between the two groups.
Go to : 
REFERENCES
1.Lee CH., Hwang JY., Oh DK., Kee MK., Oh E., An JW, et al. The burden and characteristics of tuberculosis/human immunodeficiency virus (TB/HIV) in South Korea: a study from a population database and a survey. BMC Infect Dis. 2010. 10:66.

2.Park YS., Hong SJ., Boo YK., Hwang ES., Kim HJ., Cho SH, et al. The national status of tuberculosis using nationwide medical records survey of patients with tuberculosis in Korea. Tuberc Respir Dis (Seoul). 2012. 73:48–55.

3.Kim HY., Song KS., Goo JM., Lee JS., Lee KS., Lim TH. Thoracic sequelae and complications of tuberculosis. Radiographics. 2001. 21:839–58. discussion 859-60.

4.Ellner JJ. Review: the immune response in human tuberculosis—implications for tuberculosis control. J Infect Dis. 1997. 176:1351–9.
5.Jung JW., Choi JC., Shin JW., Kim JY., Choi BW., Park IW. Pulmonary impairment in tuberculosis survivors: The Korean National Health and Nutrition Examination Survey 2008-2012. PLoS One. 2015. 10:e0141230.

6.Brausi M., Witjes JA., Lamm D., Persad R., Palou J., Colombel M, et al. A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. J Urol. 2011. 186:2158–67.

7.van der Meijden AP., Sylvester RJ., Oosterlinck W., Hoeltl W., Bono AV. EORTC Genito-Urinary Tract Cancer Group. Maintenance Bacillus Calmette-Guerin for Ta T1 bladder tumors is not associated with increased toxicity: results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group Phase III Trial. Eur Urol. 2003. 44:429–34.

8.Lamm DL., van der Meijden PM., Morales A., Brosman SA., Catalona WJ., Herr HW, et al. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol. 1992. 147:596–600.

9.Perez-Jacoiste Asin MA., Fernandez-Ruiz M., Lopez-Medrano F., Lumbreras C., Tejido A., San Juan R, et al. Bacillus Calmette-Guerin (BCG) infection following intravesical BCG administration as adjunctive therapy for bladder cancer: incidence, risk factors, and outcome in a single-institution series and review of the literature. Medicine (Baltimore). 2014. 93:236–54.
10.Yeh JJ., Neoh CA., Chen CR., Chou CY., Wu MT. A high resolution computer tomography scoring system to predict culture-positive pulmonary tuberculosis in the emergency department. PLoS One. 2014. 9:e93847.

11.Babjuk M., Burger M., Zigeuner R., Shariat SF., van Rhijn BW., Comperat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013. 64:639–53.

12.Oddens J., Brausi M., Sylvester R., Bono A., van de Beek C., van Andel G, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guerin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013. 63:462–72.
13.Lamm DL. Efficacy and safety of bacille Calmette-Guerin immunotherapy in superficial bladder cancer. Clin Infect Dis. 2000. 31(Suppl 3):S86–90.
14.Lockyer CR., Gillatt DA. BCG immunotherapy for superficial bladder cancer. J R Soc Med. 2001. 94:119–23.

15.Ratliff TL., Palmer JO., McGarr JA., Brown EJ. Intravesical Bacillus Calmette-Guerin therapy for murine bladder tumors: initiation of the response by fibronectin-mediated attachment of Bacillus Calmette-Guerin. Cancer Res. 1987. 47:1762–6.
16.Becich MJ., Carroll S., Ratliff TL. Internalization of bacille Calmette-Guerin by bladder tumor cells. J Urol. 1991. 145:1316–24.

17.Lamm DL., Blumenstein BA., Crissman JD., Montie JE., Gottesman JE., Lowe BA, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000. 163:1124–9.

18.Sylvester RJ., Brausi MA., Kirkels WJ., Hoeltl W., Calais Da Silva F., Powell PH, et al. Long-term efficacy results of EORTC genitourinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010. 57:766–73.
19.Brausi M., Oddens J., Sylvester R., Bono A., van de Beek C., van Andel G, et al. Side effects of Bacillus Calmette-Guerin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol. 2014. 65:69–76.
20.Ojea A., Nogueira JL., Solsona E., Flores N., Gomez JM., Molina JR, et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette-Guerin (27 mg) versus very low-dose bacillus Calmette-Guerin (13.5 mg) versus mitomycin C. Eur Urol. 2007. 52:1398–406.

21.Colombel M., Saint F., Chopin D., Malavaud B., Nicolas L., Rischmann P. The effect of ofloxacin on bacillus calmette-guerin induced toxicity in patients with superficial bladder cancer: results of a randomized, prospective, double-blind, placebo controlled, multicenter study. J Urol. 2006. 176:935–9.

22.Martinez-Piñeiro JA., Flores N., Isorna S., Solsona E., Sebastian JL., Pertusa C, et al. Long-term follow-up of a randomized prospective trial comparing a standard 81 mg dose of intravesical bacille Calmette-Guerin with a reduced dose of 27 mg in superficial bladder cancer. BJU Int. 2002. 89:671–80.
23.Martinez-Piñeiro JA., Martinez-Piñeiro L., Solsona E., Rodriguez RH., Gomez JM., Martin MG, et al. Has a 3-fold decreased dose of bacillus Calmette-Guerin the same efficacy against recurrences and progression of T1G3 and Tis bladder tumors than the standard dose? Results of a prospective randomized trial. J Urol. 2005. 174:1242–7.
24.Lee H., Dockrell HM., Kim DR., Floyd S., Oh SY., Lee JB, et al. The current status of BCG vaccination in young children in South Korea. Tuberc Respir Dis (Seoul). 2012. 72:374–80.

25.Lahey T., von Reyn CF. Mycobacterium bovis BCG and new vaccines for the prevention of tuberculosis. Microbiol Spectr. 2016. DOI: doi: 10.1128/microbiolspec.TNMI7-0003-2016.
Go to : 
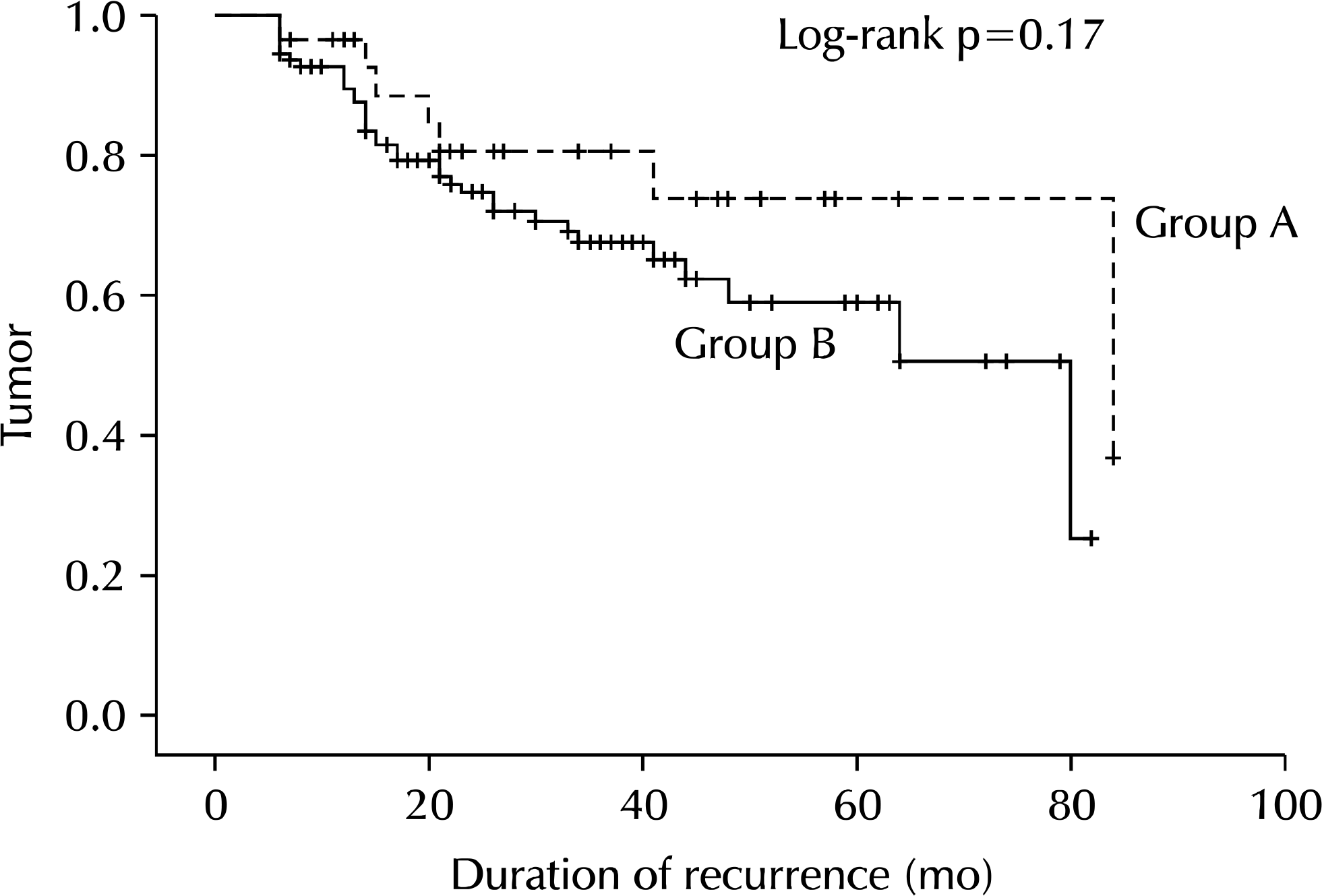 | Fig. 1.Results of log-rank test regarding recurrence-free survival in groups A and B. Group A: patients with visible sequelae of TB on chest radiography, group B: patients without visible sequelae of TB on chest radiography. |
Table 1.
Characteristics of groups A and B
Table 2.
Comparison of adverse events between groups A and B
| Adverse events | Group A (n=31) | Group B (n=111) | p-value |
|---|---|---|---|
| Cystitis symptomsa) | 10 (32.3) | 37 (33.3) | 0.910 |
| Anti-TB medication treatmentb) | 1 (3.2) | 1 (0.9) | |
| Prostate TB granulomac) | 0 (0) | 1 (0.9) | |
| Discontinuation of BCG therapy | 1 (3.2) | 3 (2.7) | 0.876 |
| Pulmonary TB | 0 (0) | 0 (0) |
Values are presented as number (%). Group A: patients with visible sequelae of TB on chest radiography, group B: patients without visible sequelae of TB on chest radiography, BCG: bacillus Calmette-Guerin, TB: tuberculosis.
a) This means persistent urinary symptoms as like dysuria, urgency, frequency and hematuria more than 24 hours after BCG instillation.
Table 3.
Characteristics of patients with cystitis symptoms between groups A and B
Table 4.
Results of urine analysis between groups A and B, from the patients with cystitis symptoms




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download