Abstract
Purpose:
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) causes pain and urinary symptoms that involve the prostate and/or other parts of the male. We analyzed the clinical outcomes of medication and microwave thermotherapy.
Materials and Methods:
A total of 132 patients with CP/CPPS for at least 3 months were assigned to one of the three study groups (group A: medication; group B: thermotherapy; group C: combination therapy). The NIH-CPSI was recorded at baseline, and at weeks 2, 4, 8, and 12 post-therapy. EPS was evaluated, and semen analysis was performed to assess the changes in prostatic inflammation. Moreover, patient satisfaction questionnaire was completed.
Results:
Comparisons between groups A and B, as well as between groups B and C showed no significant changes in pain, quality of life, and total scores. At week 12, group C, when compared with group A, had a significantly improved voiding score (4.19±3.02 vs. 2.71±2.30, p=0.019) and EPS (12.47±15.91 vs. 3.73±4.82, p=0.003). At week 4, the patient satisfaction score in group C was significantly different from that in other groups (p=0.043), but there was no difference at week 12 (p>0.05). There was no statistically significant difference in laboratory test results, PSA, and prostate volume between the three groups at baseline and week 12. Complications of thermotherapy resolved with conservative management.
REFERENCES
1.Nickel JC., Krieger JN., McNaughton-Collins M., Anderson RU., Pontari M., Shoskes DA, et al. Alfuzosin and symptoms of chronic prostatitis-chronic pelvic pain syndrome. N Engl J Med. 2008. 359:2663–73.

2.Woo YN. Prostatitis. Korean J Urol. 1994. 35:575–85.
3.Lee KC., Jung PB., Park HS., Whang JH., Lee JG. Transurethral needle ablation for chronic nonbacterial prostatitis. BJU Int. 2002. 89:226–9.

4.Gottsch HP., Yang CC., Berger RE. A pilot study of botulinum toxin A for male chronic pelvic pain syndrome. Scand J Urol Nephrol. 2011. 45:72–6.

5.Zimmermann R., Cumpanas A., Miclea F., Janetschek G. Extracorporeal shock wave therapy for the treatment of chronic pelvic pain syndrome in males: a randomised, double-blind, placebo-controlled study. Eur Urol. 2009. 56:418–24.

6.Hurwitz MD., Kaplan ID., Svensson GK., Hynynen K., Hansen MS. Feasibility and patient tolerance of a novel transrectal ultrasound hyperthermia system for treatment of prostate cancer. Int J Hyperthermia. 2001. 17:31–7.

7.Stawarz B., Zielinski H., Szmigielski S., Rappaport E., Debicki P., Petrovich Z. Transrectal hyperthermia as palliative treatment for advanced adenocarcinoma of prostate and studies of cell-mediated immunity. Urology. 1993. 41:548–53.

8.Montorsi F., Guazzoni G., Colombo R., Galli L., Bergamaschi F., Rigatti P. Transrectal microwave hyperthermia for advanced prostate cancer: long-term clinical results. J Urol. 1992. 148:342–5.

9.Apolikhin OI., Voronovitskii VD., Gonsales EN. Experience with application of ALMGP-01 device for local transrectal microwave hyperthermia of the prostate in the treatment of chronic prostatitis. Urologiia. 2010. 5:39–41.
10.Kehinde EO., Abul F. Transurethral microwave thermotherapy for treatment of benign prostatic hyperplasia: a preliminary assessment of the Prostalund feedback treatment machine. Med Princ Pract. 2005. 14:272–6.

11.Montorsi F., Guazzoni G., Rigatti P., Pizzini G., Miani A. Is there a role for transrectal microwave hyperthermia in the treatment of benign prostatic hyperplasia? A critical review of a six-year experience. J Endourol. 1995. 9:333–7.

12.Montorsi F., Galli L., Guazzoni G., Colombo R., Bulfamante G., Barbieri L, et al. Transrectal microwave hyperthermia for benign prostatic hyperplasia: long-term clinical, pathological and ultrastructural patterns. J Urol. 1992. 148:321–5.

13.Giubilei G., Mondaini N., Minervini A., Saieva C., Lapini A., Serni S, et al. Physical activity of men with chronic prostatitis/chronic pelvic pain syndrome not satisfied with conventional treatments—could it represent a valid option? The physical activity and male pelvic pain trial: a double-blind, randomized study. J Urol. 2007. 177:159–65.

14.Shoskes DA., Hakim L., Ghoniem G., Jackson CL. Long-term results of multimodal therapy for chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2003. 169:1406–10.

15.Tuğcu V., Tasci AI., Fazlioglu A., Gurbuz G., Ozbek E., Sahin S, et al. A placebo-controlled comparison of the efficiency of triple- and monotherapy in category III B chronic pelvic pain syndrome (CPPS). Eur Urol. 2007. 51:1113–7. discussion 1118.

16.Lee KS., Choi JD. Chronic prostatitis: approaches for best management. Korean J Urol. 2012. 53:69–77.

17.Kastner C., Hochreiter W., Huidobro C., Cabezas J., Miller P. Cooled transurethral microwave thermotherapy for intractable chronic prostatitis—results of a pilot study after 1 year. Urology. 2004. 64:1149–54.

18.Choi NG., Soh SH., Yoon TH., Song MH. Clinical experience with transurethral microwave thermotherapy for chronic nonbacterial prostatitis and prostatodynia. J Endourol. 1994. 8:61–4.

19.Nickel JC., Sorensen R. Transurethral microwave thermotherapy for nonbacterial prostatitis: a randomized double-blind sham controlled study using new prostatitis specific assessment questionnaires. J Urol. 1996. 155:1950–5.

20.Thakkinstian A., Attia J., Anothaisintawee T., Nickel JC. -Blockers, antibiotics and anti-inflammatories have a role in the management of chronic prostatitis/chronic pelvic pain syndrome. BJU Int. 2012. 110:1014–22.

21.Nickel JC., Roehrborn CG., O'Leary MP., Bostwick DG., Somerville MC., Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol. 2008. 54:1379–84.

22.Nickel JC., Roehrborn CG., O'leary MP., Bostwick DG., Somerville MC., Rittmaster RS. Examination of the relationship between symptoms of prostatitis and histological inflammation: baseline data from the REDUCE chemoprevention trial. J Urol. 2007. 178:896–900. discussion 900-1.

23.Stancik I., Plas E., Juza J., Pfluger H. Effect of antibiotic therapy on interleukin-6 in fresh semen and postmasturbation urine samples of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2008. 72:336–9.

24.Liu L., Li Q., Han P., Li X., Zeng H., Zhu Y, et al. Evaluation of interleukin-8 in expressed prostatic secretion as a reliable biomarker of inflammation in benign prostatic hyperplasia. Urology. 2009. 74:340–4.

25.Lotti F., Corona G., Mancini M., Filimberti E., Degli Innocenti S., Colpi GM, et al. Ultrasonographic and clinical correlates of seminal plasma interleukin-8 levels in patients attending an andrology clinic for infertility. Int J Androl. 2011. 34:600–13.

26.He L., Wang Y., Long Z., Jiang C. Clinical significance of IL-2, IL-10, and TNF-alpha in prostatic secretion of patients with chronic prostatitis. Urology. 2010. 75:654–7.
27.Paulis G., Conti E., Voliani S., Bertozzi MA., Sarteschi ML., Menchini Fabris F. Evaluation of the cytokines in genital secretions of patients with chronic prostatitis. Arch Ital Urol Androl. 2003. 75:179–86.
Fig. 2.
Study flow diagram. Group A: medical therapy, group B: microwave thermotherapy, group C: medication and microwave thermotherapy.
Fig. 3.
Changes of pain, voiding, quality of life (QoL), and total score. Group A: medical therapy, group B: microwave thermotherapy, group C: medication and microwave thermotherapy, NIH-CPSI: The National Institutes of Health Chronic Prostatitis Symptom Index.
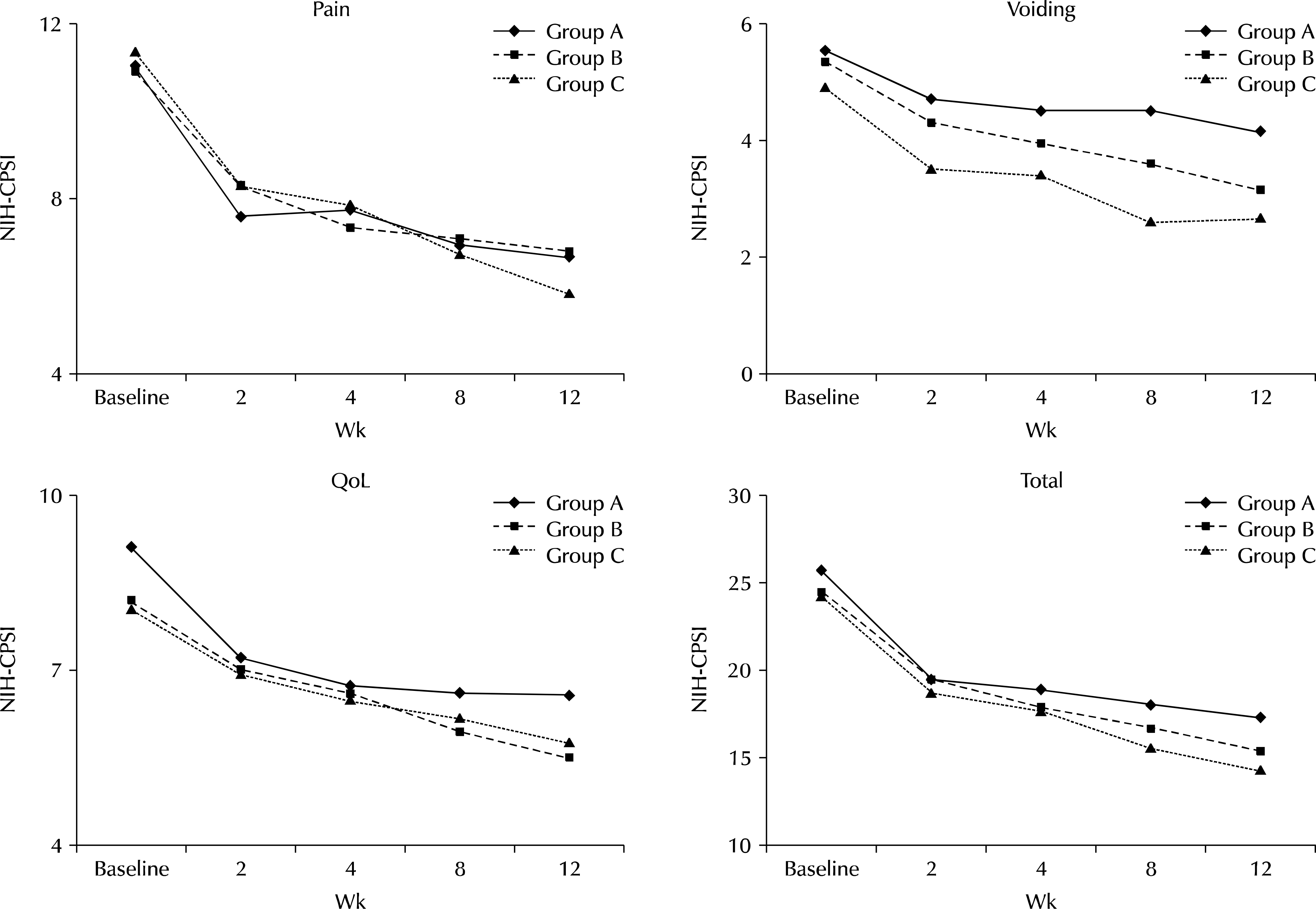
Table 1.
Patient characteristics
Table 2.
NIH-CPSI score at the baseline and at week 12
Table 3.
Changes of patient satisfaction questionnaire




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download