Abstract
Purpose:
This study aims to investigate the specific pathogenic properties or virulent determinant characteristics of uropathogenic Escherichia coli (UPEC) as bacterial adherence in tissue culture cells in vitro and the pathogenicity in animal model in vivo.
Materials and Methods:
Thirty strains of E. coli were isolated from urine of patients with acute pyelonephritis. Four cell lines—HeLa cells, HEp-2 cells, A-498 cells, and J-82 cells—were used for bacterial adherence assay. Histologic examination and scanning electron microscopy examination of pyelonephritis or cystitis, which was caused by E. coli, in mice was performed.
Results:
Sixteen (53.3%) strains of E. coli appeared to adhere to at least one or more kinds of four cell lines, and seven strains were able to adhere to all four cell lines. All of the tested E. coli strains were adhered to the mouse bladder and kidneys. The number of bacteria colonized in the kidney was greater than that of bladders in the following 5 strains of E. coli: TME104, TME107, TME113, TME306, and TME119. There was no difference in the number of bacteria colonized in the bladder and kidneys in the aspects of adherence patterns to tissue culture cells.
Conclusions:
Although there was no best choice cell lines in the adherence assay to identify the adherence patterns, combined assays of in vitro cell culture and in vivo model of mouse urinary tract infection appeared to be efficient methods to investigate the role of bacterial adherence in the pathogenesis of UPEC.
REFERENCES
1.Terlizzi ME., Gribaudo G., Maffei ME. UroPathogenic Escherichia coli (UPEC) infections: virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front Microbiol. 2017. 8:1566.

2.Luthje P., Brauner A. Virulence factors of uropathogenic E. coli and their interaction with the host. Adv Microb Physiol. 2014. 65:337–72.
3.Boll EJ., Struve C., Boisen N., Olesen B., Stahlhut SG., Krogfelt KA. Role of enteroaggregative Escherichia coli virulence factors in uropathogenesis. Infect Immun. 2013. 81:1164–71.

4.Cravioto A., Gross RJ., Scitland SW., Rowe B. An ahresive factor found in strains of Escherichia coli belonging to the traditional infantile enteropathogenic serotypes. Curr Microbiol. 1979. 3:95–9.
5.Nataro JP., Scaletsky IC., Kaper JB., Levine MM., Trabulsi LR. Plasmid-mediated factors conferring diffuse and localized adherence of enteropathogenic Escherichia coli. Infect Immun. 1985. 48:378–83.

6.Scaletsky IC., Silva ML., Trabulsi LR. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984. 45:534–6.

7.Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Eden C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983. 40:265–72.

8.Linder H., Engberg I., Baltzer IM., Jann K., Svanborg-Eden C. Induction of inflammation by Escherichia coli on the mucosal level: requirement for adherence and endotoxin. Infect Immun. 1988. 56:1309–13.

9.Srivastava R., Agarwal J., Srivastava S., Mishra B. Role of special pathogenicity versus prevalence theory in pathogenesis of acute cystitis caused by Escherichia coli. J Med Microbiol. 2014. 63:1038–43.

10.Svanborg Eden C., Hausson S., Jodal U., Lidin-Janson G., Lincoln K., Linder H, et al. Host-parasite interaction in the urinary tract. J Infect Dis. 1988. 157:421–6.
11.Roberts JA. Norwich-Eaton lectureship. Pathogenesis of nonobstructive urinary tract infections in children. J Urol. 1990. 144:475–9.
12.See WA., Williams RD. Urothelial injury and clotting cascade activation: common denominators in particulate adherence to urothelial surfaces. J Urol. 1992. 147:541–8.

13.Brauner A., Katouli M., Tullus K., Jacobson SH. Production of cytotoxic necrotizing factor, verocytotoxin and haemolysin by pyelonephritogenic Escherichia coli. Eur J Clin Microbiol Infect Dis. 1990. 9:762–7.
14.Totsika M., Moriel DG., Idris A., Rogers BA., Wurpel DJ., Phan MD, et al. Uropathogenic Escherichia coli mediated urinary tract infection. Curr Drug Targets. 2012. 13:1386–99.

15.Hagberg L., Engberg I., Freter R., Lam J., Olling S., Svanborg Eden C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983. 40:273–83.

Fig. 1.
Light micrographs showing 3 types of adherence pattern in HeLa, HEp-2, A-498, and J-82 cells. DA denotes a diffuse adherence to the TME104 strain on the dispersed surface of the cultured cells. LA represents a localized adherence of the TME303 strain to the localized areas of cultured cells and formed to particulate microcolonies. AA denotes an aggregative adherence of TME114 strain to the cultured cells. Note the characteristic aggregates of bacteria that showed “stacked-brick” appearance. They bound aggregately to both of cultured cells and bottom of cell culture dish (×500).

Fig. 2.
Inflammation of the mouse bladder (A) and kidney (B) caused by bacterial infections. H&E, ×100.

Fig. 3.
Scanning electron micrographs showing diffuse adherence (DA) of TME104, localized adherence (LA) of TME303, and aggregative adherence (AA) of TME114 Escherichia coli strains adhered to J-82 cells. Magnification, ×1,500.
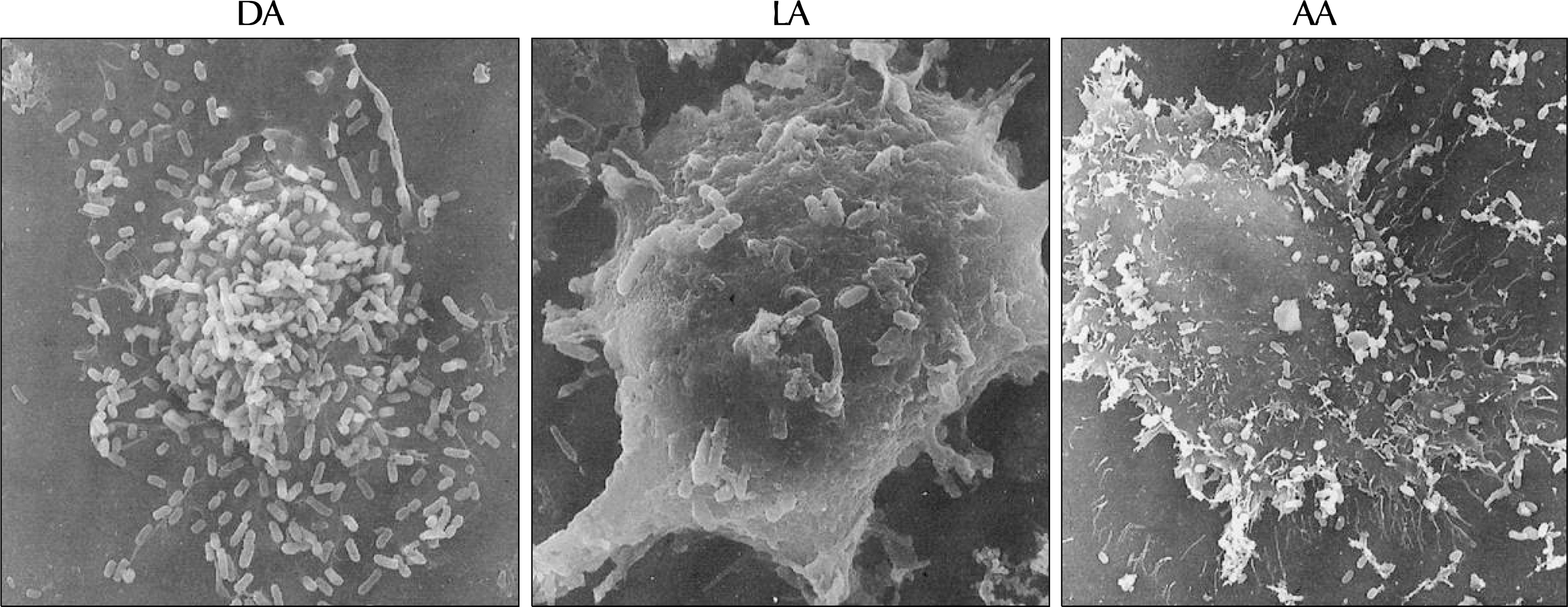
Fig. 4.
Scanning electron micrographs showing TME116 (A, B), TME303 (C, D), and TME114 (E, F) Escherichia coli strains adhering to mouse urinary bladder. Magnification, A, C, E: ×2,200; B, D, F: ×15,000. DA: diffuse adherence, LA: localized adherence, AA: aggregative adherence, NA: non-adherence.

Table 1.
Adherence of Escherichia coli strains to the host cells
Table 2.
In vivo adherence of Escherichia coli strains to mouse bladder and kidneys
| Strain no. | Adherence pattern | No. of bacteria (/0.1 ml of tissue homogenate)a) | |
|---|---|---|---|
| Bladder | Kidneys | ||
| TME104 | DA | 5.5×107 | 1.7×108 |
| TME108 | DA | 9.4×104 | 3.5×104 |
| TME116 | DA | 2.1×105 | 6.7×104 |
| TME107 | LA | 3.5×104 | 9.5×104 |
| TME113 | LA | 3.2×104 | 1.3×105 |
| TME303 | LA | 7.1×106 | 2.2×103 |
| TME114 | AA | 7.4×108 | 5.2×108 |
| TME306 | AA | 3.1×105 | 3.3×105 |
| TME307 | AA | 1.2×104 | 6.0×102 |
| TME115 | NA | 9.2×105 | 7.2×105 |
| TME117 | NA | 5.1×105 | 6.3×103 |
| TME119 | NA | 1.2×104 | 6.1×106 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download