Abstract
Purpose:
CC-chemokine ligand 11 (CCL11; eotaxin-1), an eosinophil chemoattractant chemokine, has been proposed as a serum marker for prostate cancer (PCa) by two research groups. We investigated the usefulness of CCL11 in diagnosing prostatic diseases, such as benign prostatic hyperplasia (BPH) and PCa.
Materials and Methods:
CCL11 was measured in the sera of 139 men with BPH, 44 men with PCa, and 45 control men attending an outpatient health-screening clinic. A commercial enzyme-linked immunosorbent assay kit was used to measure CCL11.
Results:
CCL11 concentrations were significantly higher in men with BPH and PCa than in normal men (72.9±3.15 and 80.0±4.91 pg/ml vs. 57.6±8.24). In addition, a receiver operating characteristic (ROC) analysis of serum CCL11 levels showed that the areas under the ROC curves were 0.661 (p=0.001) and 0.654 (p=0.012) for BPH and PCa, respectively, compared with normal men.
REFERENCES
1.Matthews AN., Friend DS., Zimmermann N., Sarafi MN., Luster AD., Pearlman E, et al. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci U S A. 1998. 95:6273–8.

2.Solari R., Pease JE., Begg M. Chemokine receptors as therapeutic targets: why aren't there more drugs? Eur J Pharmacol. 2015. 746:363–7.

3.Agarwal M., He C., Siddiqui J., Wei JT., Macoska JA. CCL11 (eotaxin-1): a new diagnostic serum marker for prostate cancer. Prostate. 2013. 73:573–81.

4.Heidegger I., Hofer J., Luger M., Pichler R., Klocker H., Horninger W, et al. Is Eotaxin-1 a serum and urinary biomarker for prostate cancer detection and recurrence? Prostate. 2015. 75:1904–9.

5.Bianchi-Frias D., Vakar-Lopez F., Coleman IM., Plymate SR., Reed MJ., Nelson PS. The effects of aging on the molecular and cellular composition of the prostate microenvironment. PLoS One. 2010. 5:DOI: doi: 10.1371/journal.pone.0012501.

6.Wei JT., Calhoun E., Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005. 173:1256–61.

8.St Sauver JL., Jacobsen SJ. Inflammatory mechanisms associated with prostatic inflammation and lower urinary tract symptoms. Curr Prostate Rep. 2008. 6:67–73.

9.Sfanos KS., De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012. 60:199–215.

10.Penna G., Mondaini N., Amuchastegui S., Degli Innocenti S., Carini M., Giubilei G, et al. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007. 51:524–33.

11.Bouraoui Y., Ricote M., Garcia-Tunon I., Rodriguez-Berriguete G., Touffehi M., Rais NB, et al. Pro-inflammatory cytokines and prostate-specific antigen in hyperplasia and human prostate cancer. Cancer Detect Prev. 2008. 32:23–32.

12.Macoska JA., Begley LA., Dunn RL., Siddiqui J., Wei JT., Sarma AV. Pilot and feasibility study of serum chemokines as markers to distinguish prostatic disease in men with low total serum PSA. Prostate. 2008. 68:442–52.

13.Zhu F., Liu P., Li J., Zhang Y. Eotaxin-1 promotes prostate cancer cell invasion via activation of the CCR3-ERK pathway and upregulation of MMP-3 expression. Oncol Rep. 2014. 31:2049–54.

14.Kelly RS., Vander Heiden MG., Giovannucci E., Mucci LA. Metabolomic biomarkers of prostate cancer: prediction, diagnosis, progression, prognosis, and recurrence. Cancer Epidemiol Biomarkers Prev. 2016. 25:887–906.

15.Collin SM., Martin RM., Metcalfe C., Gunnell D., Albertsen PC., Neal D, et al. Prostate-cancer mortality in the USA and UK in 1975-2004: an ecological study. Lancet Oncol. 2008. 9:445–52.

16.Miller DC., Hollenbeck BK. Missing the mark on prostate-specific antigen screening. JAMA. 2011. 306:2719–20.

17.Cazares LH., Drake RR., Esquela-Kirscher A., Lance RS., Semmes OJ., Troyer DA. Molecular pathology of prostate cancer. Cancer Biomark. 2010. 9:441–59.

18.Martin NE., Mucci LA., Loda M., Depinho RA. Prognostic determinants in prostate cancer. Cancer J. 2011. 17:429–37.

19.Netto GJ., Cheng L. Emerging critical role of molecular testing in diagnostic genitourinary pathology. Arch Pathol Lab Med. 2012. 136:372–90.

20.Salami SS., Schmidt F., Laxman B., Regan MM., Rickman DS., Scherr D, et al. Combining urinary detection of TMPRSS2: ERG and PCA3 with serum PSA to predict diagnosis of prostate cancer. Urol Oncol. 2013. 31:566–71.
21.Gerber BO., Zanni MP., Uguccioni M., Loetscher M., Mackay CR., Pichler WJ, et al. Functional expression of the eotaxin receptor CCR3 in T lymphocytes co-localizing with eosinophils. Curr Biol. 1997. 7:836–43.

22.Uguccioni M., Mackay CR., Ochensberger B., Loetscher P., Rhis S., LaRosa GJ, et al. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest. 1997. 100:1137–43.

23.Sallusto F., Mackay CR., Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997. 277:2005–7.

24.Levina V., Nolen BM., Marrangoni AM., Cheng P., Marks JR., Szczepanski MJ, et al. Role of eotaxin-1 signaling in ovarian cancer. Clin Cancer Res. 2009. 15:2647–56.

25.Waddell A., Ahrens R., Tsai YT., Sherrill JD., Denson LA., Stein-brecher KA, et al. Intestinal CCL11 and eosinophilic inflammation is regulated by myeloid cell-specific RelA/p65 in mice. J Immunol. 2013. 190:4773–85.

26.Nolen BM., Lokshin AE. Targeting CCL11 in the treatment of ovarian cancer. Expert Opin Ther Targets. 2010. 14:157–67.

27.Chao PZ., Chou CM., Chen CH. Plasma RANTES and eotaxin levels are correlated with the severity of chronic rhinosinusitis. Eur Arch Otorhinolaryngol. 2012. 269:2343–8.

28.Geiger SM., Jardim-Botelho A., Williams W., Alexander N., Diemert DJ., Bethony JM. Serum CCL11 (eotaxin-1) and CCL17 (TARC) are serological indicators of multiple helminth infections and are driven by Schistosoma mansoni infection in humans. Trop Med Int Health. 2013. 18:750–60.
29.Byers LA., Holsinger FC., Kies MS., William WN., El-Naggar AK., Lee JJ, et al. Serum signature of hypoxia-regulated factors is associated with progression after induction therapy in head and neck squamous cell cancer. Mol Cancer Ther. 2010. 9:1755–63.

30.Johrer K., Zelle-Rieser C., Perathoner A., Moser P., Hager M., Ramoner R, et al. Up-regulation of functional chemokine receptor CCR3 in human renal cell carcinoma. Clin Cancer Res. 2005. 11:2459–65.

31.Egler RA., Li Y., Dang TA., Peters TL., Leung E., Huang S, et al. An integrated proteomic approach to identifying circulating biomarkers in high-risk neuroblastoma and their potential in relapse monitoring. Proteomics Clin Appl. 2011. 5:532–41.

Fig. 1.
Serum CC-chemokine ligand 11 (CCL11) levels (pg/ml) in men without prostatic disease (normal) and patients with benign prostatic hyperplasia (BPH) and prostate cancer (PCa). (A) CCL11 differed significantly between the three group (Kruskal-Wallis test, ∗p-value=0). (B) CCL11 levels are significantly higher in prostatic disease (BPH or PCa) than in normal men (Mann-Whitney test, ∗p-value=0.001). (C) CCL11 levels in patients with prostate cancers with different Gleason scores (GS).
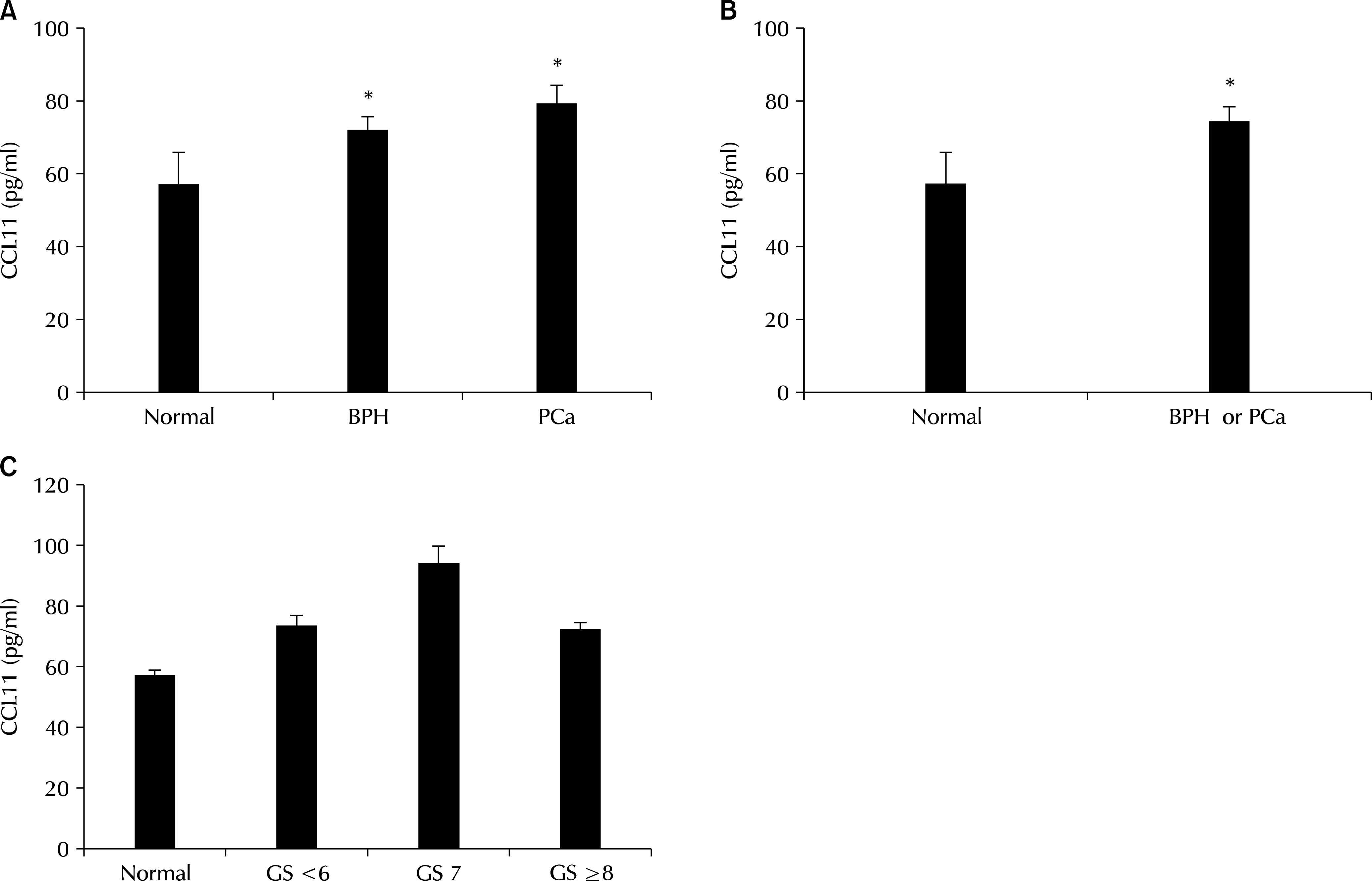
Fig. 2.
Receiver operating characteristic (ROC) curves for CC-chemokine ligand 11 (CCL11). (A) ROC curves distinguishing between benign prostatic hyperplasia (BPH) and no disease. The optimal cutoff of the CCL11 levels for distinguishing BPH from control (no disease) was determined as 53.1955 using the ROC curve (sensitivity=0.691, 1-specificity=0.422). Area under the curve (AUC) was 0.661, and 95% confidence interval (CI) was 0.560-0.762, p=0.001. (B) ROC curve distinguishing between prostate cancer (PCa) and no disease. The optimal cutoff of the CCL11 levels for distinguishing PCa from control (no disease) was determined as 52.6195 using the ROC curve (sensitivity=0.659, 1-specificity=0.467). AUC was 0.654, and 95% CI was 0.540-0.768, p=0.012. (C) ROC curve distinguishing between prostatic disease and no disease. The optimal cutoff of the CCL11 levels for distinguishing prostatic diseases from no disease was determined as 53.1955 using the ROC curve (sensitivity=0.683, 1-specificity=0.422). AUC was 0.659, and 95% CI was 0.562-0.757, p=0.001.
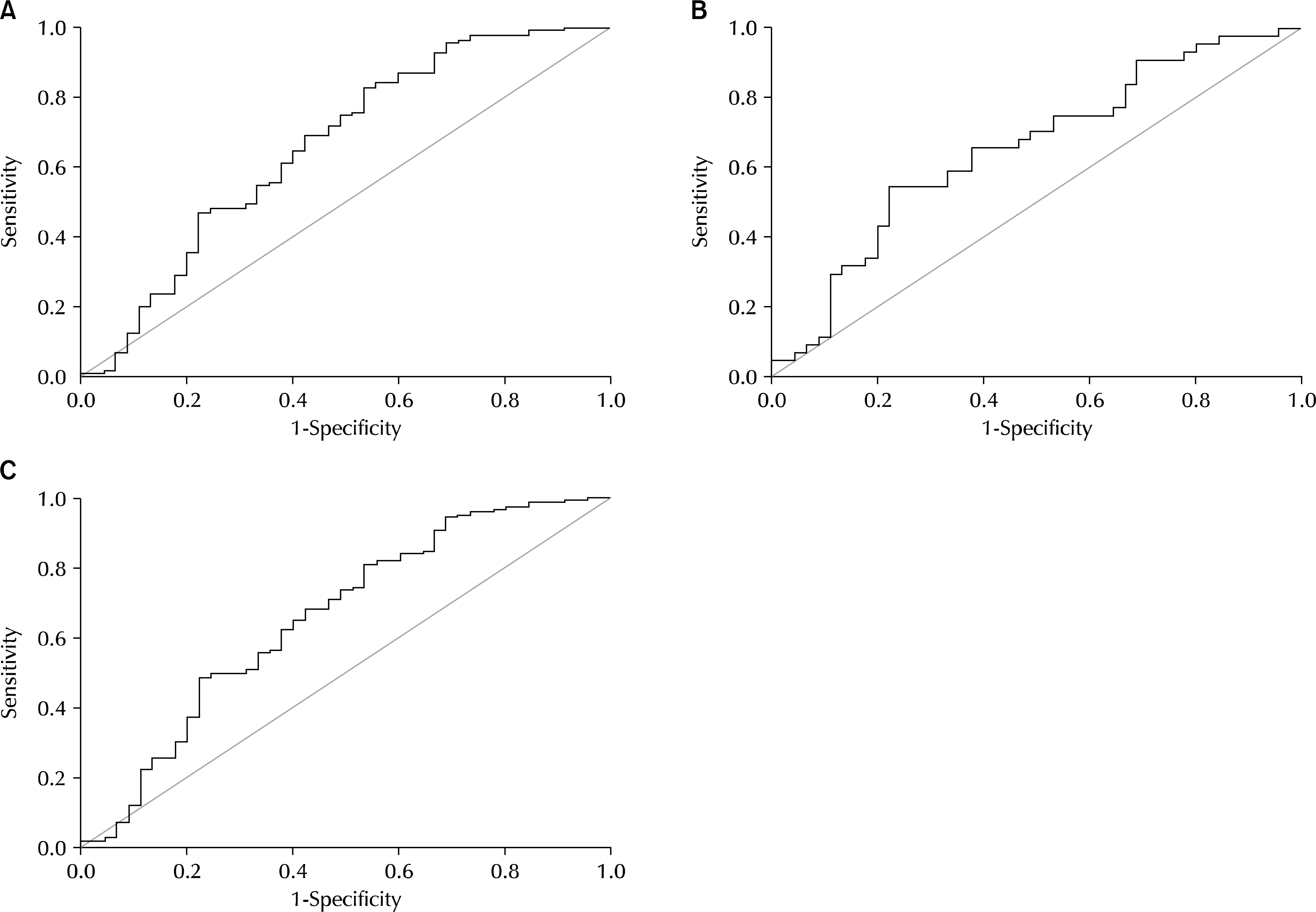
Table 1.
Patient demographic, clinical and CCL11 data
All the data are expressed as means±standard errors except for total numbers. PSA differed significantly between the three group (Kruskal-Wallis test, p<0.0001). Forty-four with prostate cancer included 8 patients with Gleason scores (GS) <6, 17 patients with GS of 7 and 19 patients with GS of 8 or more.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download