Abstract
The most common type of prostatitis is category III, also known as chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). The current National Institutes of Health definition of CP/CPPS includes genitourinary pain with or without voiding symptoms in the absence of uropathogenic bacteria, as detected by standard microbiological methods, or other identifiable causes such as malignancy. Many different etiologies and mechanisms of pathogenesis of CP/CPPS have been proposed with a suggested role for immunological, neurological, endocrine, and psychological factors. We examined the data supporting the role of each of these areas and also examined the possible interrelationship of these factors in producing the symptoms of CP/CPPS. Prostatitis types IIIa and IIIb are classified according to the presence of pain without concurrent presence of bacteria; however, it is becoming more evident that, although levels of bacteria are not directly associated with levels of pain, the presence of bacteria might act as the initiating factor that drives primary activation of mast-cell-mediated inflammation in the prostate. The gate control theory provides a neurologic basis for the influence of both somatic and psychological factors on pain. Acceptance of chronic pain as a diagnosis may be difficult for the clinician and patient, however it is an important concept in the care of CP/CPPS, which enables the use of pain-directed therapies. Management of CP/CPPS will remain challenging; however, this review provides a better understanding of the condition and improved management strategies based on the newest evidence and concepts available.
Go to : 
REFERENCES
1.Krieger JN., Nyberg L Jr., Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999. 282:236–7.

2.Collins MM., Meigs JB., Barry MJ., Walker Corkery E., Gio-vannucci E., Kawachi I. Prevalence and correlates of prostatitis in the health professionals follow-up study cohort. J Urol. 2002. 167:1363–6.

3.Mehik A., Hellström P., Lukkarinen O., Sarpola A., Järvelin M. Epidemiology of prostatitis in Finnish men: a population-based cross-sectional study. BJU Int. 2000. 86:443–8.

4.Schaeffer AJ., Landis JR., Knauss JS., Propert KJ., Alexander RB., Litwin MS, et al. Chronic Prostatitis Collaborative Research Network Group. Demographic and clinical characteristics of men with chronic prostatitis: the national institutes of health chronic prostatitis cohort study. J Urol. 2002. 168:593–8.

5.Nickel JC., Alexander RB., Schaeffer AJ., Landis JR., Knauss JS., Propert KJ. Chronic Prostatitis Collaborative Research Network Study Group. Leukocytes and bacteria in men with chronic prostatitis/chronic pelvic pain syndrome compared to asymptomatic controls. J Urol. 2003. 170:818–22.

6.Leskinen MJ., Vainionp R., Syrjnen S., Leppilahti M., Marttila T., Kylml T, et al. Herpes simplex virus, cytomegalovirus, and papillomavirus DNA are not found in patients with chronic pelvic pain syndrome undergoing radical prostatectomy for localized prostate cancer. Urology. 2003. 61:397–401.

7.Leskinen MJ., Rantakokko-Jalava K., Manninen R., Leppilahti M., Marttila T., Kylmälä T; T, et al. Negative bacterial polymerase chain reaction (PCR) findings in prostate tissue from patients with symptoms of chronic pelvic pain syndrome (CPPS) and localized prostate cancer. Prostate. 2003. 55:105–10.

8.Shoskes DA., Shahed AR. Detection of bacterial signal by 16S rRNA polymerase chain reaction in expressed prostatic secretions predicts response to antibiotic therapy in men with chronic pelvic pain syndrome. Tech Urol. 2000. 6:240–2.
9.Kanbay M., Gur G., Akcay S., Yilmaz U. Helicobacter pylori seroprevalence in patients with chronic bronchitis. Respir Med. 2005. 99:1213–6.

10.Mendall MA., Goggin PM., Molineaux N., Levy J., Toosy T., Strachan D, et al. Relation of Helicobacter pylori infection and coronary heart disease. Br Heart J. 1994. 71:437–9.

11.Rebora A., Drago F., Picciotto A. Helicobacter pylori in patients with rosacea. Am J Gastroenterol. 1994. 89:1603–4.
12.Karatas OF., Turkay C., Bayrak O., Cimentepe E., Unal D. Helicobacter pylori seroprevalence in patients with chronic prostatitis: a pilot study. Scand J Urol Nephrol. 2010. 44:91–4.

13.Geramoutsos I., Gyftopoulos K., Perimenis P., Thanou V., Liagka D., Siamblis D, et al. Clinical correlation of prostatic lithiasis with chronic pelvic pain syndromes in young adults. Eur Urol. 2004. 45:333–7. discussion 337-8.

14.Shoskes DA., Thomas KD., Gomez E. Anti-nanobacterial therapy for men with chronic prostatitis/chronic pelvic pain syndrome and prostatic stones: preliminary experience. J Urol. 2005. 173:474–7.

16.Zhou Z., Hong L., Shen X., Rao X., Jin X., Lu G, et al. Detection of nanobacteria infection in type III prostatitis. Urology. 2008. 71:1091–5.

17.Weidner W., Schiefer HG., Krauss H., Jantos C., Friedrich HJ., Altmannsberger M. Chronic prostatitis: a thorough search for etiologically involved microorganisms in 1,461 patients. Infection. 1991. 19(Suppl 3):S119–25.

18.Pontari MA., McNaughton-Collins M., O'leary MP., Calhoun EA., Jang T., Kusek JW, et al. CPCRN Study Group. A case-control study of risk factors in men with chronic pelvic pain syndrome. BJU Int. 2005. 96:559–65.

19.Rudick CN., Berry RE., Johnson JR., Johnston B., Klumpp DJ., Schaeffer AJ, et al. Uropathogenic Escherichia coli induces chronic pelvic pain. Infect Immun. 2011. 79:628–35.
20.True LD., Berger RE., Rothman I., Ross SO., Krieger JN. Prostate histopathology and the chronic prostatitis/chronic pelvic pain syndrome: a prospective biopsy study. J Urol. 1999. 162:2014–8.

21.Nickel JC., Roehrborn CG., O'Leary MP., Bostwick DG., Somerville MC., Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol. 2008. 54:1379–84.

22.Schaeffer AJ., Knauss JS., Landis JR., Propert KJ., Alexander RB., Litwin MS, et al. Chronic Prostatitis Collaborative Research Network Study Group. Leukocyte and bacterial counts do not correlate with severity of symptoms in men with chronic prostatitis: the National Institutes of Health Chronic Prostatitis Cohort Study. J Urol. 2002. 168:1048–53.

23.Hochreiter WW., Nadler RB., Koch AE., Campbell PL., Ludwig M., Weidner W, et al. Evaluation of the cytokines interleukin 8 and epithelial neutrophil activating peptide 78 as indicators of inflammation in prostatic secretions. Urology. 2000. 56:1025–9.

24.Nadler RB., Koch AE., Calhoun EA., Campbell PL., Pruden DL., Bennett CL, et al. IL-1beta and TNF-alpha in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J Urol. 2000. 164:214–8.
25.Desireddi NV., Campbell PL., Stern JA., Sobkoviak R., Chuai S., Shahrara S, et al. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha as possible biomarkers for the chronic pelvic pain syndrome. J Urol. 2008. 179:1857–61. discussion 1861-2.
26.Thumbikat P., Shahrara S., Sobkoviak R., Done J., Pope RM., Schaeffer AJ. Prostate secretions from men with chronic pelvic pain syndrome inhibit proinflammatory mediators. J Urol. 2010. 184:1536–42.

27.Miller LJ., Fischer KA., Goralnick SJ., Litt M., Burleson JA., Albertsen P, et al. Nerve growth factor and chronic prostatitis/chronic pelvic pain syndrome. Urology. 2002. 59:603–8.

28.Watanabe T., Inoue M., Sasaki K., Araki M., Uehara S., Monden K, et al. Nerve growth factor level in the prostatic fluid of patients with chronic prostatitis/chronic pelvic pain syndrome is correlated with symptom severity and response to treatment. BJU Int. 2011. 108:248–51.

29.Woolf CJ., Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991. 44:293–9.
30.Davis SN., Maykut CA., Binik YM., Amsel R., Carrier S. Tenderness as measured by pressure pain thresholds extends beyond the pelvis in chronic pelvic pain syndrome in men. J Sex Med. 2011. 8:232–9.

31.Bennett MI., Simpson KH. Gabapentin in the treatment of neuropathic pain. Palliat Med. 2004. 18:5–11.

32.Rose MA., Kam PC. Gabapentin: pharmacology and its use in pain management. Anaesthesia. 2002. 57:451–62.

33.Guay DR. Pregabalin in neuropathic pain: a more "pharma-ceutically elegant" gabapentin? Am J Geriatr Pharmacother. 2005. 3:274–87.

34.Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg. 2007. 105:1805–15.

35.Chiechio S., Zammataro M., Caraci F., Rampello L., Copani A., Sabato AF, et al. Pregabalin in the treatment of chronic pain: an overview. Clin Drug Investig. 2009. 29:203–13.
36.Tripp DA., Curtis Nickel J., Landis JR., Wang YL., Knauss JS. CPCRN Study Group. Predictors of quality of life and pain in chronic prostatitis/chronic pelvic pain syndrome: findings from the National Institutes of Health Chronic Prostatitis Cohort Study. BJU Int. 2004. 94:1279–82.

37.Tripp DA., Nickel JC., Shoskes D., Koljuskov A. A 2-year follow-up of quality of life, pain, and psychosocial factors in patients with chronic prostatitis/chronic pelvic pain syndrome and their spouses. World J Urol. 2013. 31:733–9.

38.Zhang GX., Bai WJ., Xu T., Wang XF. A preliminary evaluation of the psychometric profiles in Chinese men with chronic prostatitis/chronic pelvic pain syndrome. Chin Med J (Engl). 2011. 124:514–8.
39.Ahn SG., Kim SH., Chung KI., Park KS., Cho SY., Kim HW. Depression, anxiety, stress perception, and coping strategies in Korean military patients with chronic prostatitis/chronic pelvic pain syndrome. Korean J Urol. 2012. 53:643–8.

40.Anderson RU., Orenberg EK., Morey A., Chavez N., Chan CA. Stress induced hypothalamus-pituitary-adrenal axis responses and disturbances in psychological profiles in men with chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2009. 182:2319–24.

41.Anderson RU., Orenberg EK., Chan CA., Morey A., Flores V. Psychometric profiles and hypothalamic-pituitary-adrenal axis function in men with chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2008. 179:956–60.

42.Beck AT., Epstein N., Brown G., Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988. 56:893–7.

43.Clemens JQ., Brown SO., Calhoun EA. Mental health diagnoses in patients with interstitial cystitis/painful bladder syndrome and chronic prostatitis/chronic pelvic pain syndrome: a case/control study. J Urol. 2008. 180:1378–82.

44.Chung SD., Lin HC. Association between chronic prostatitis/chronic pelvic pain syndrome and anxiety disorder: a population-based study. PLoS One. 2013. 8:e64630.

45.Tripp DA., Nickel JC., Wang Y., Litwin MS., McNaughton-Collins M., Landis JR, et al. National Institutes of Health-Chronic Prostatitis Collaborative Research Network (NIH-CPCRN) Study Group. Catastrophizing and pain-contingent rest predict patient adjustment in men with chronic prostatitis/chronic pelvic pain syndrome. J Pain. 2006. 7:697–708.

46.Nickel JC., Tripp DA., Chuai S., Litwin MS., McNaughton-Collins M., Landis JR, et al. NIH-CPCRN Study Group. Psychosocial variables affect the quality of life of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome. BJU Int. 2008. 101:59–64.

47.Hu JC., Link CL., McNaughton-Collins M., Barry MJ., McKinlay JB. The association of abuse and symptoms suggestive of chronic prostatitis/chronic pelvic pain syndrome: results from the Boston Area Community Health survey. J Gen Intern Med. 2007. 22:1532–7.

48.Spanos C., Pang X., Ligris K., Letourneau R., Alferes L., Alexacos N, et al. Stress-induced bladder mast cell activation: implications for interstitial cystitis. J Urol. 1997. 157:669–72.

49.Keast JR., Kepper ME. Differential regulation of trkA and p75 in noradrenergic pelvic autonomic ganglion cells after deafferentation of their cholinergic neighbours. Eur J Neurosci. 2001. 13:211–20.

50.Meusburger SM., Keast JR. Testosterone and nerve growth factor have distinct but interacting effects on structure and neurotransmitter expression of adult pelvic ganglion cells in vitro. Neuroscience. 2001. 108:331–40.

51.Di Franco M., Iannuccelli C., Valesini G. Neuroendocrine immunology of fibromyalgia. Ann N Y Acad Sci. 2010. 1193:84–90.

52.McEwen BS., Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism. 2010. 59(Suppl 1):S9–15.

53.Dimitrakov J., Joffe HV., Soldin SJ., Bolus R., Buffington CA., Nickel JC. Adrenocortical hormone abnormalities in men with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2008. 71:261–6.

54.Kroeger KM., Carville KS., Abraham LJ. The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol. 1997. 34:391–9.
55.Turner DM., Williams DM., Sankaran D., Lazarus M., Sinnott PJ., Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997. 24:1–8.

56.Shoskes DA., Albakri Q., Thomas K., Cook D. Cytokine polymorphisms in men with chronic prostatitis/chronic pelvic pain syndrome: association with diagnosis and treatment response. J Urol. 2002. 168:331–5.

57.Riley DE., Krieger JN. X Chromosomal short tandem repeat polymorphisms near the phosphoglycerate kinase gene in men with chronic prostatitis. Biochim Biophys Acta. 2002. 1586:99–107.

58.Arisan ED., Arisan S., Kiremit MC., Tiğli H., Caşkurlu T., Palavan-Unsal N, et al. Manganese superoxide dismutase polymorphism in chronic pelvic pain syndrome patients. Prostate Cancer Prostatic Dis. 2006. 9:426–31.
59.Dimitrakov J., Guthrie D. Genetics and phenotyping of urological chronic pelvic pain syndrome. J Urol. 2009. 181:1550–7.

60.Klein RJ., Zeiss C., Chew EY., Tsai JY., Sackler RS., Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005. 308:385–9.

61.Nickel JC. Prostatitis and related conditions, orchitis, and epididymitis. Wein AJ, Kavoussi LR, Partin AW, Novick AC, Peters CA, editors. editors.Campbell-Walsh urology. 10th ed.Philadelphia: Saunders;2012. 333.

62.Murphy SF., Schaeffer AJ., Thumbikat P. Immune mediators of chronic pelvic pain syndrome. Nat Rev Urol. 2014. 11:259–69.

63.Rapkin AJ. Neuroanatomy, neurophysiology, and neuropharmacology of pelvic pain. Clin Obstet Gynecol. 1990. 33:119–29.
Go to : 
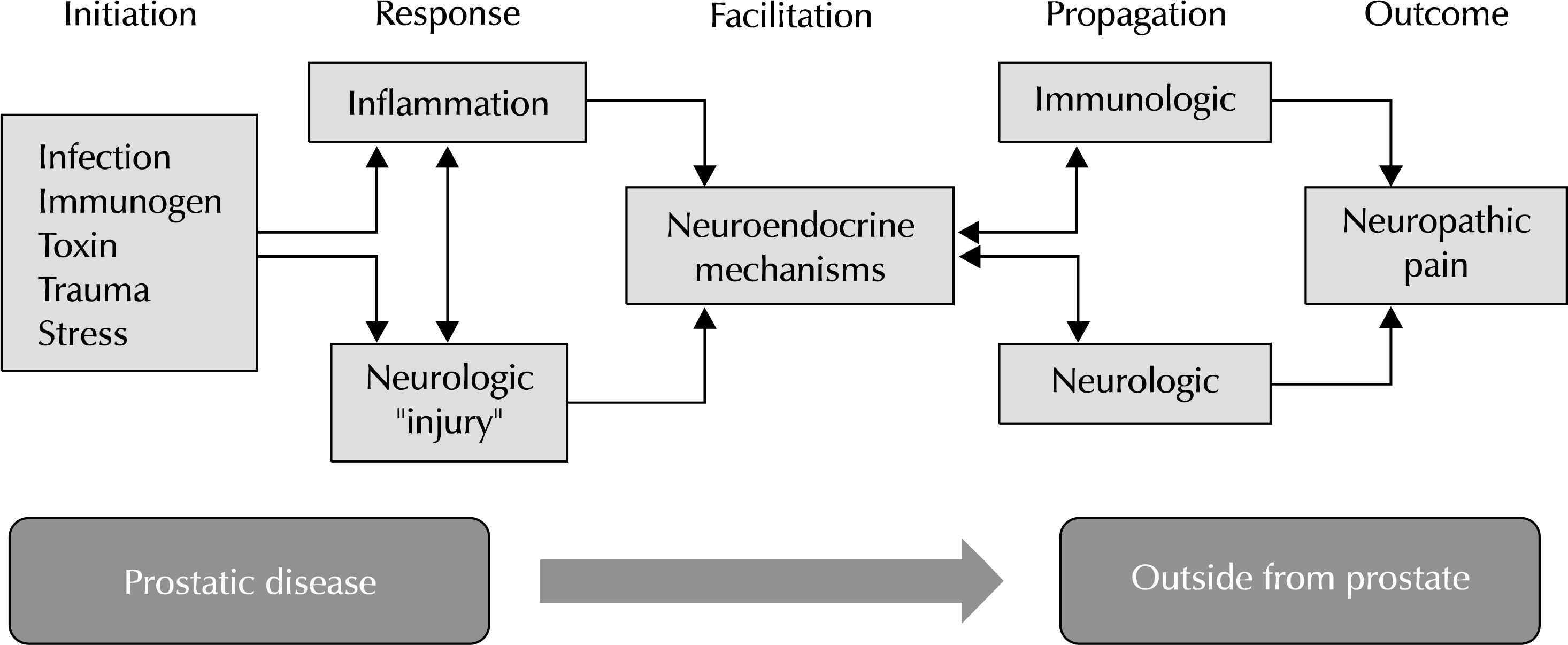 | Fig. 1.Hypothetical scenario that could potentially involve most of the proposed and interrelated causes in chronic prostatitis/chronic pelvic pain syndrome. |
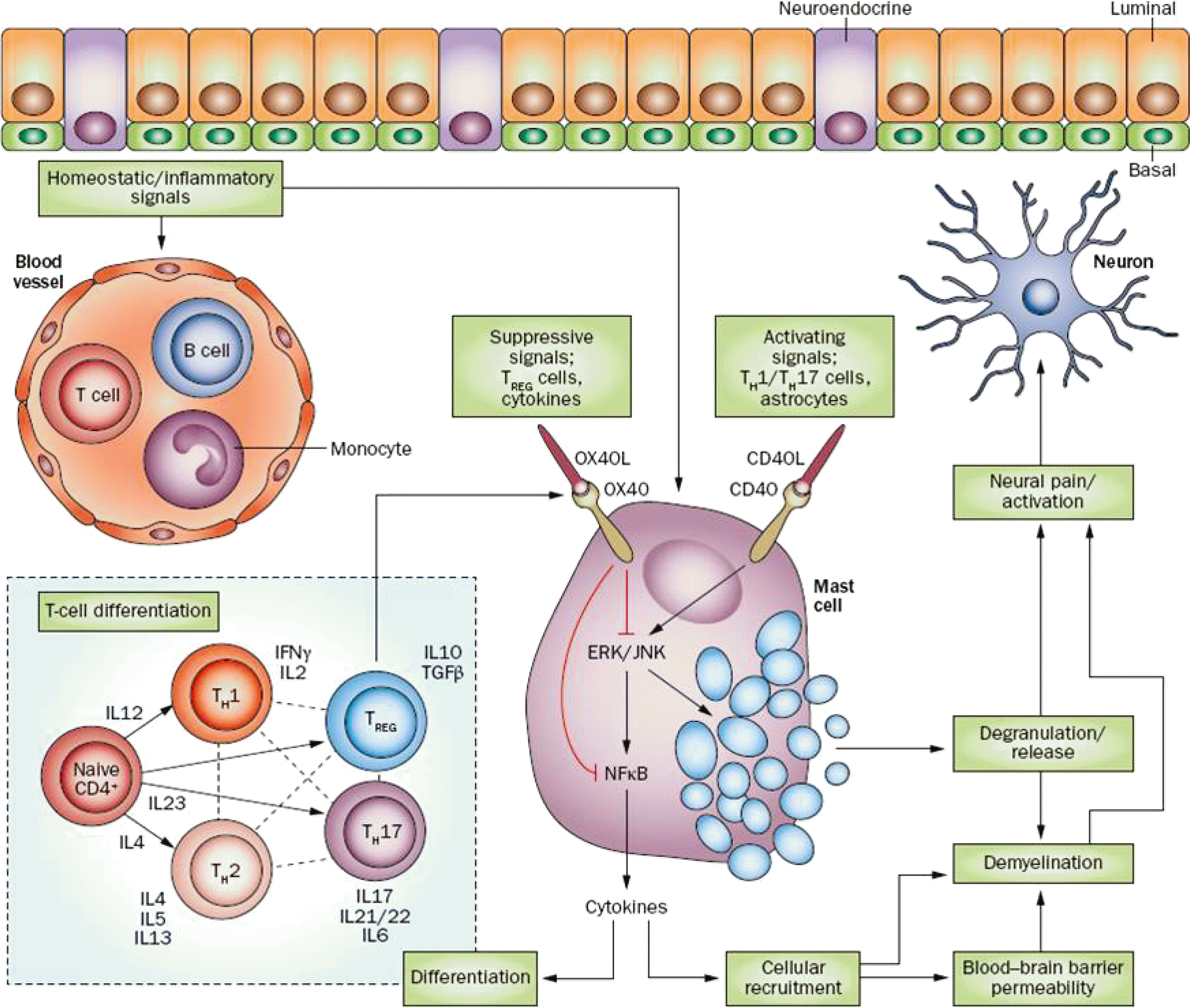 | Fig. 2.Hypothetical model of chronic pelvic pain syndrome. Intercellular homeostatic signaling demonstrating differentiation of CD4 T-cells in a context-dependent manner, regulation of mast cell activation by direct interaction (for example, through OX40 receptor), and activation of mast cells and subsequent deactivation, as well as how these signaling cascades interact to stimulate neurons. Reproduced from the article of Murphy et al. Nat Rev Urol 2014;11:259-69 [62] with permission. Treg: regulatory T, ERK: extracellular signal-regulated kinases, JNK: c-Jun N-terminal kinase, NFB: nuclear factor kappa B, IFN: interferon gamma, IL: interleukin, TGF: transforming growth factor beta. |
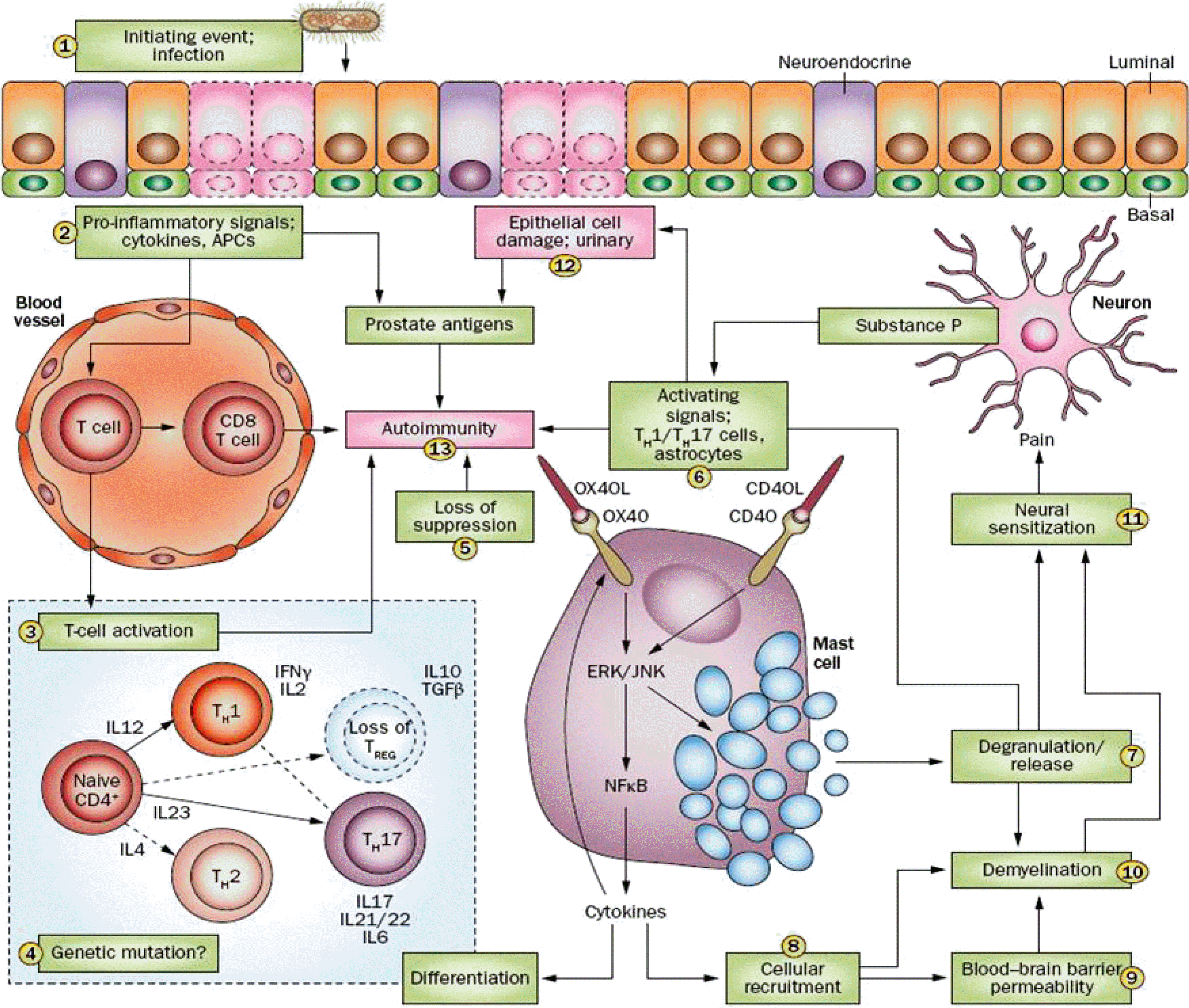 | Fig. 3.Hypothetical schematic representation of a modulated immune system in chronic prostatitis/chronic pelvic pain syndrome. An initiating event, such as bacterial infection, drives prostatic epithelial cell damage (1) and promotes the secretion and activation of proinflammatory cytokines, chemokines and presentation of antigens via antigen-presenting cells (APCs) (2). These signaling cascades result in CD4 T-cell activation, which is initially of Th1-type (interferon gamma, IFN) but Th17 activation is also implicated in the pathway, possibly at later stages of chronic pain development (3), followed by a loss of interleukin (IL)10-secreting suppressive regulatory T (Treg) cells and a skewing towards Th1/17 responses (4). The loss of suppression of mast cells as a result of unchecked T-cell activation then results in a positive feedback loop in the mast cell (5, 6), resulting in degranulation and release of proteases such as tryptase, chymase, and allergy mediators such as histamine (7), and secretion of cytokines (such as IL6, IL17, tumor necrosis factor-, and IL6), resulting in the recruitment of inflammatory cells (8) and potentially disrupting the blood–brain barrier (9) and demyelinating neurons (10). Taken together, these processes result in neuronal activation and sensitization (11). The mast cell mediates these events and positive feedback loops enhance these processes. Further epithelial cell damage is one consequence of such increased mast cell activity (12). Prostate antigens generated from damage to the epithelium in the presence of an activated CD4 T-cell response, with unchecked mast cell degranulation and increased numbers of CD8 T-cells can result in the development of autoimmunity, which further exacerbates these mechanisms (13). Reproduced from the article of Murphy et al. Nat Rev Urol 2014;11:259-69 [62] with permission. TGF: transforming growth factor beta, ERK: extracellular signal-regulated kinases, JNK: c-Jun N-terminal kinase, NFB: nuclear factor kappa B. |
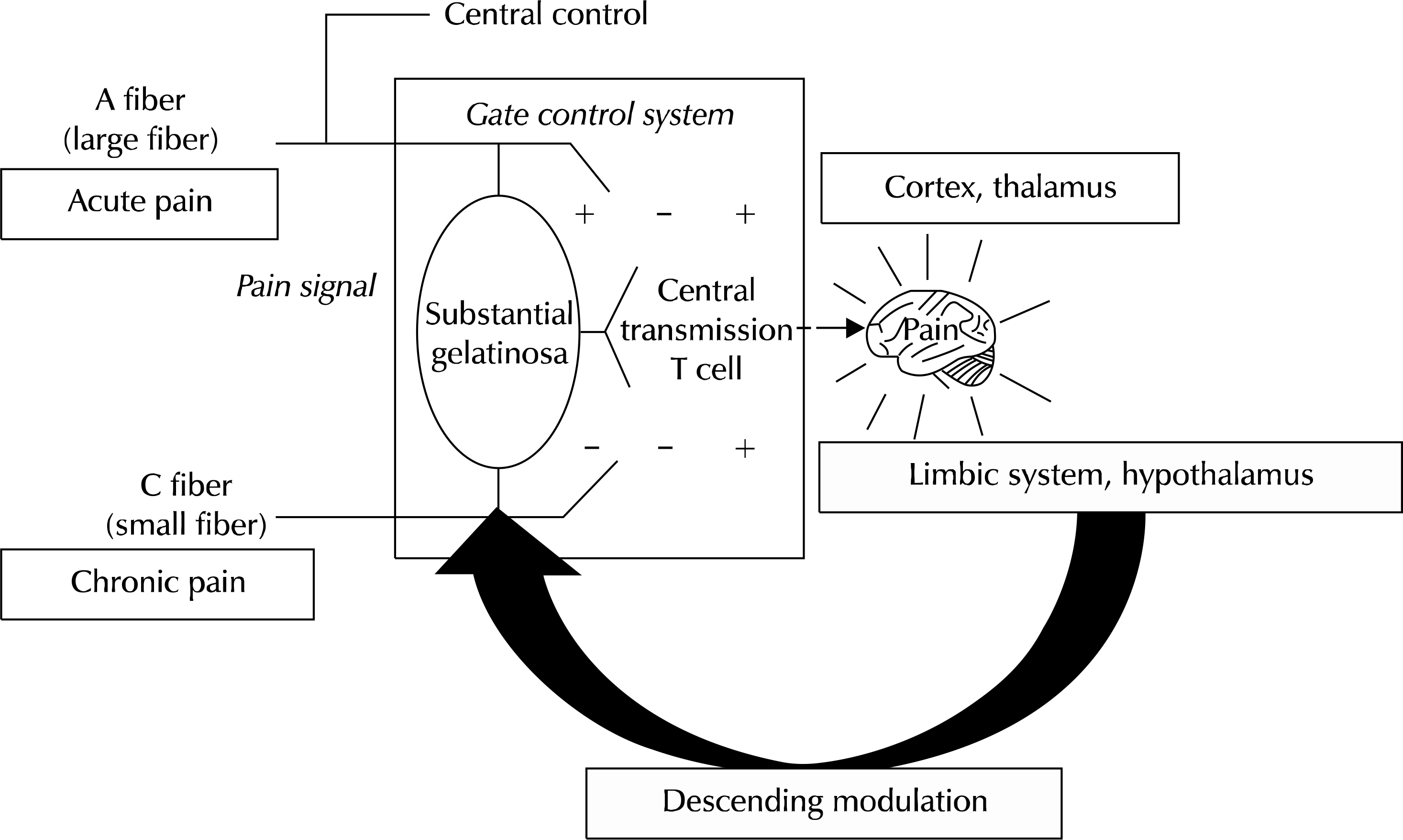 | Fig. 4.The gate control theory of pain. This theory is based on the following propositions. (1) The transmission of nerve impulses from afferent fibers to spinal cord transmission (T) cells is modulated by a spinal mechanism. Gating mechanism in the dorsal horn. (2) The spinal gating mechanism is influenced by the relative amount of activity in large-diameter (L) and small-diameter (S) fibers: activity in large fibers tends to inhibit transmission (close the gate) while small-fiber activity tends to facilitate transmission (open the gate). (3) The spinal gating mechanism is influenced by nerve impulses that descend from the brain. (4) A specialized system of large-diameter, rapidly conducting fibers (the central control trigger) activates selective cognitive processes that then influence, by way of descending fibers, the modulating properties of the spinal gating mechanism. (5) When the output of the spinal cord transmission (T) cells exceeds a critical level, it activates the action system-those neural areas that underlie the complex, sequential patterns of behavior and experience characteristic of pain. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download