Abstract
Purpose:
Many benign prostatic hyperplasia (BPH) patients were accompanied by pelvic pain apart from urinary symptoms. Therefore, we evaluate the treatment outcomes of alpha-blockers via a change of international prostate symptom score (IPSS) according to pain score of the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI).
Materials and Methods:
A total of 356 male patients with BPH from March 2011 to May 2014 were analyzed retrospectively. Prostate specific antigen, prostate volume, IPSS, NIH-CPSI, international index of erectile function (IIEF-5), and uroflowmetry were collected. Patients were categorized according to 2 groups based on the presence and severity of pain and baseline characteristics and treatment outcomes were analyzed.
Results:
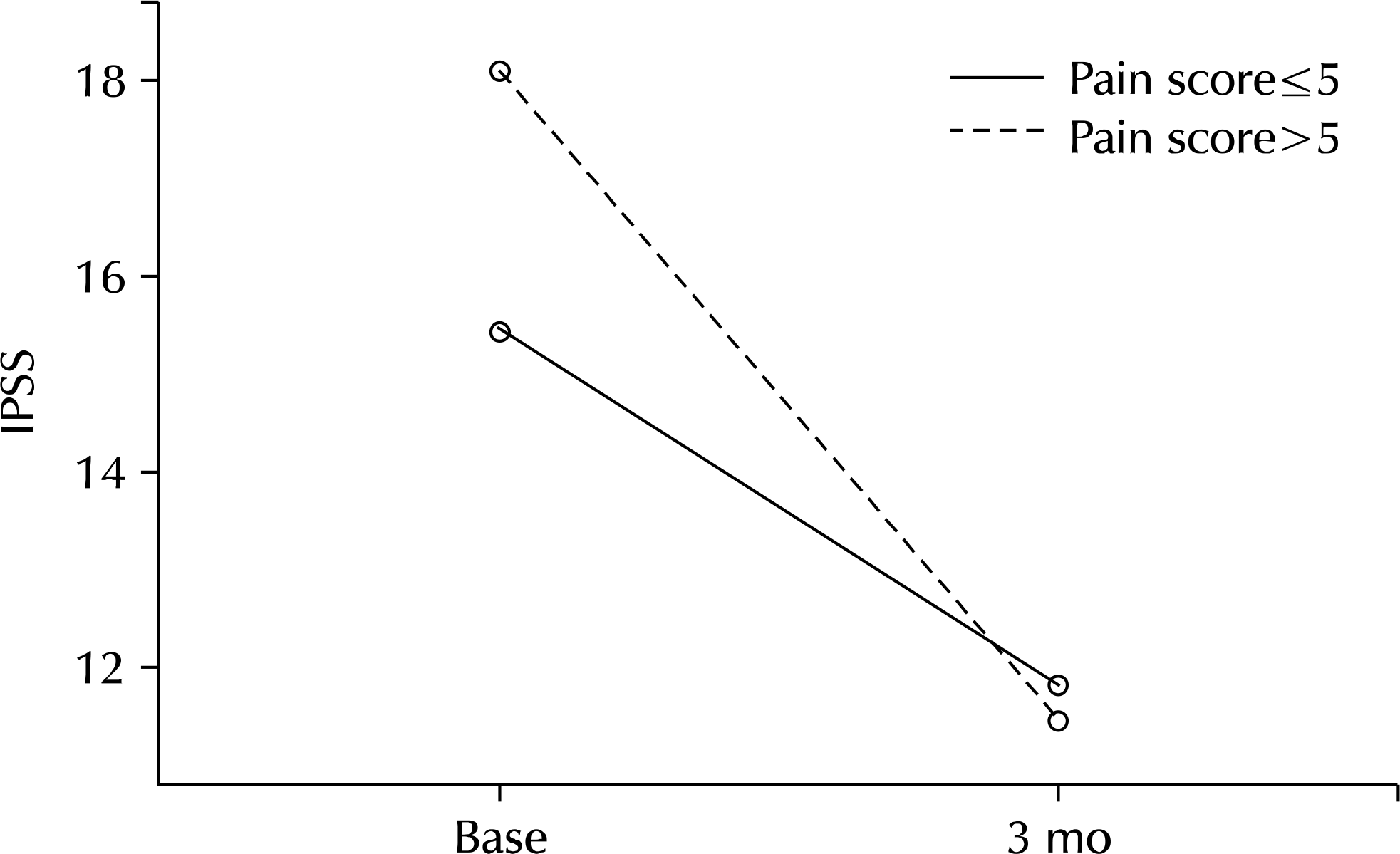
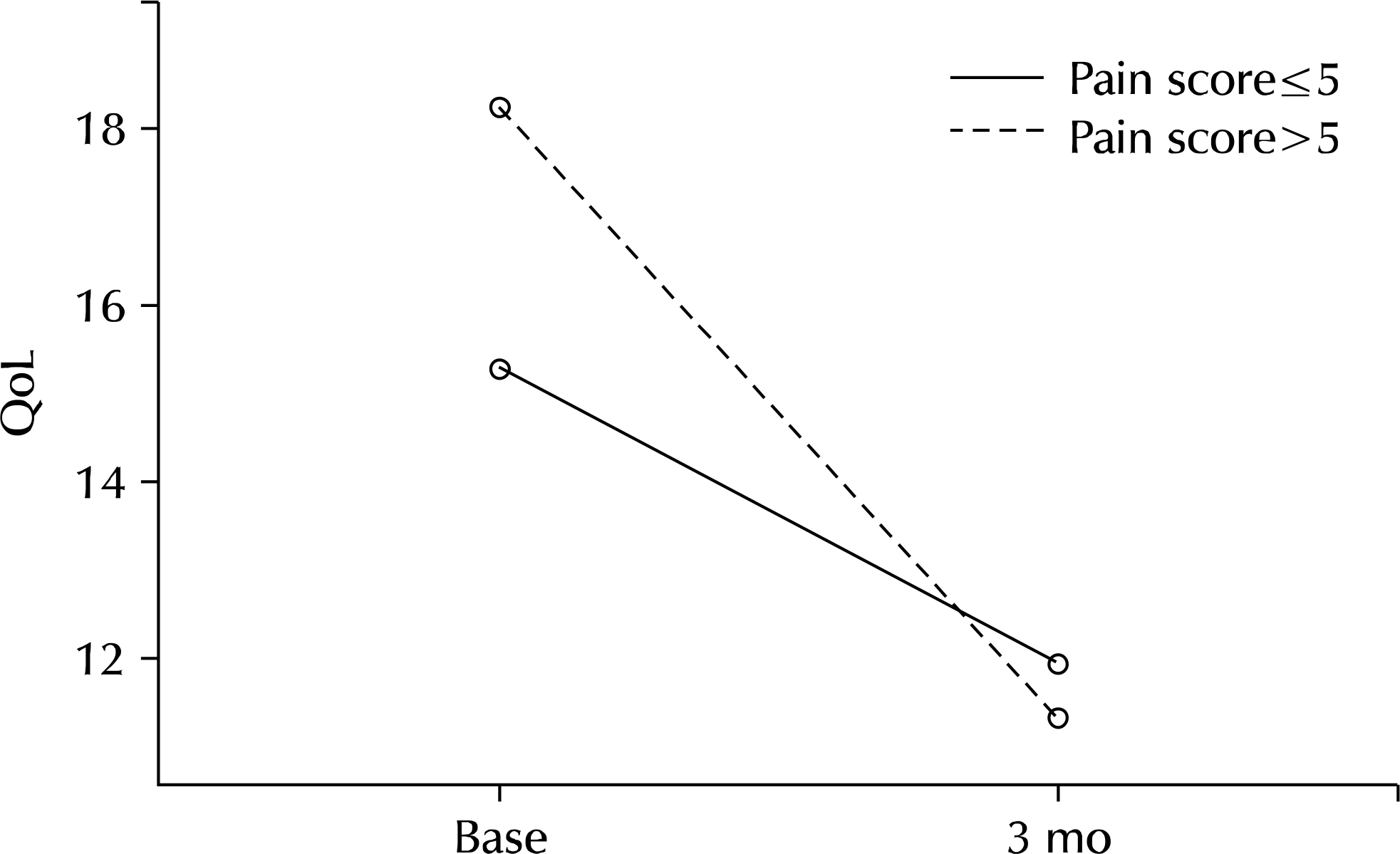
Two hundred twenty-nine patients (64.3%) reported pain/discomfort on NIH-CPSI. Mean IPSS, mean voiding symptoms, mean storage symptoms on IPSS, and mean IIEF-5 showed a significant difference in groups 1A and 1B. Logistic regression analysis showed that NIH-CPSI pain score was a significant predictive factor for severe IPSS (odds ratio, 2.830; 95% confidence interval, 1.307-6.129). After treatment for 3 months, improvement of IPSS, voiding symptoms, storage symptoms, and quality of life was observed in all groups (p=0.001, p<0.001, p=0.026, p<0.001, p<0.001, p<0.001, p<0.001, p<0.001). Group 2B (pain score>5) showed greater improvement of symptoms and statistically significant difference compared with group 2A (pain score ≤5) (p=0.029, p=0.026).
Go to : 
REFERENCES
1.Abrams P., Cardozo L., Fall M., Griffiths D., Rosier P., Ulmsten U, et al. Standardisation Sub-committee of the International Continence Society. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002. 21:167–78.

2.Wei JT., Calhoun E., Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005. 173:1256–61.

3.Collins MM., Meigs JB., Barry MJ., Walker Corkery E., Gio-vannucci E., Kawachi I. Prevalence and correlates of prostatitis in the health professionals follow-up study cohort. J Urol. 2002. 167:1363–6.

4.Garraway WM., Collins GN., Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991. 338:469–71.

5.De Nunzio C., Kramer G., Marberger M., Montironi R., Nelson W., Schröder F, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol. 2011. 60:106–17.

6.Briganti A., Capitanio U., Suardi N., Gallina A., Salonia A., Bianchi M, et al. Benign prostatic hyperplasia and its aetiologies. Eur Urol Suppl. 2009. 8:865–71.

7.Krieger JN., Lee SW., Jeon J., Cheah PY., Liong ML., Riley DE. Epidemiology of prostatitis. Int J Antimicrob Agents. 2008. 31(Suppl 1):S85–90.

8.Vallancien G., Emberton M., Harving N., van Moorselaar RJ. Alf-One Study Group. Sexual dysfunction in 1,274 European men suffering from lower urinary tract symptoms. J Urol. 2003. 169:2257–61.

9.Nickel JC., Roehrborn CG., O'leary MP., Bostwick DG., Somerville MC., Rittmaster RS. Examination of the relationship between symptoms of prostatitis and histological inflammation: baseline data from the REDUCE chemoprevention trial. J Urol. 2007. 178:896–900.

10.Nickel JC., Elhilali M., Vallancien G. ALF-ONE Study Group. Benign prostatic hyperplasia (BPH) and prostatitis: prevalence of painful ejaculation in men with clinical BPH. BJU Int. 2005. 95:571–4.

11.Braun MH., Sommer F., Haupt G., Mathers MJ., Reifenrath B., Engelmann UH. Lower urinary tract symptoms and erectile dysfunction: co-morbidity or typical "Aging Male" symptoms? Results of the "Cologne Male Survey". Eur Urol. 2003. 44:588–94.

12.Rosen R., Altwein J., Boyle P., Kirby RS., Lukacs B., Meuleman E, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7). Eur Urol. 2003. 44:637–49.

13.Collins MM., Stafford RS., O'Leary MP., Barry MJ. How common is prostatitis? A national survey of physician visits. J Urol. 1998. 159:1224–8.

14.Chung WS., Nehra A., Jacobson DJ., Roberts RO., Rhodes T., Girman CJ, et al. Lower urinary tract symptoms and sexual dysfunction in community-dwelling men. Mayo Clin Proc. 2004. 79:745–9.

15.Lepor H., Williford WO., Barry MJ., Haakenson C., Jones K. Veterans Affairs Cooperative Studies Benign Prostatic Hyperplasia Study Group. The impact of medical therapy on bother due to symptoms, quality of life and global outcome, and factors predicting response. J Urol. 1998. 160:1358–67.

16.Nickel JC., Fradet Y., Boake RC., Pommerville PJ., Perreault JP., Afridi SK, et al. Efficacy and safety of finasteride therapy for benign prostatic hyperplasia: results of a 2-year randomized controlled trial (the PROSPECT study). PROscar Safety Plus Efficacy Canadian Two year Study. CMAJ. 1996. 155:1251–9.
17.McConnell JD., Bruskewitz R., Walsh P., Andriole G., Lieber M., Holtgrewe HL, et al. Finasteride Long-Term Efficacy and Safety Study Group. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med. 1998. 338:557–63.

18.Roehrborn CG., Boyle P., Nickel JC., Hoefner K., Andriole G. ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002. 60:434–41.

19.Cheah PY., Liong ML., Yuen KH., Teh CL., Khor T., Yang JR, et al. Terazosin therapy for chronic prostatitis/chronic pelvic pain syndrome: a randomized, placebo controlled trial. J Urol. 2003. 169:592–6.

20.Mehik A., Alas P., Nickel JC., Sarpola A., Helström PJ. Alfuzosin treatment for chronic prostatitis/chronic pelvic pain syndrome: a prospective, randomized, double-blind, placebo-controlled, pilot study. Urology. 2003. 62:425–9.

21.Nickel JC., Narayan P., McKay J., Doyle C. Treatment of chronic prostatitis/chronic pelvic pain syndrome with tamsulosin: a randomized double blind trial. J Urol. 2004. 171:1594–7.

22.Kwon YK., Choe MS., Seo KW., Park CH., Chang HS., Kim BH, et al. The effect of intraprostatic chronic inflammation on benign prostatic hyperplasia treatment. Korean J Urol. 2010. 51:266–70.

23.Cantoro U., Catanzariti F., Lacetera V., Quaresima L., Muzzonigro G., Polito M. Comparison of tamsulosin vs tamsulosin/sildenafil effectiveness in the treatment of erectile dysfunction in patients affected by type III chronic prostatitis. Arch Ital Urol Androl. 2013. 85:109–12.

24.Lacquaniti S., Destito A., Servello C., Candidi MO., Weir JM., Brisinda G, et al. Terazosine and tamsulosin in non bacterial prostatitis: a randomized placebo-controlled study. Arch Ital Urol Androl. 1999. 71:283–5.
25.de la Rosette JJ., Karthaus HF., van Kerrebroeck PE., de Boo T., Debruyne FM. Research in 'prostatitis syndromes': the use of alfuzosin (a new alpha 1-receptor-blocking agent) in patients mainly presenting with micturition complaints of an irritative nature and confirmed urodynamic abnormalities. Eur Urol. 1992. 22:222–7.
Go to : 
Table 1.
Baseline characteristics (n=356)
Table 2.
Risk factor for National Institutes of Health-Chronic Prostatitis Symptom Index pain score
Table 3.
Comparison of treatment outcomes before and after treatment in each group
Table 4.
The changes of subjective and objective parameters between 2 groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download