Abstract
Background:
The autophagy is the major route for lysosomal degradation of misfolded protein aggregates and oxidative cell components. We hypothesized that rapamycin (autophagy enhancer) would prolong the survival of motor neuron and suppress the disease progression in amyotrophic lateral sclerosis (ALS).
Methods:
A total of 24 transgenic mice harboring the human G93A mutated SOD1 gene were used. The clinical status involving rotarod test and survival, and biochemical study of ALS mice model were evaluated.
Results:
The onset of symptoms was significantly delayed in the rapamycin administration group compared with the control group. However, after the clinical symptom developed, the rapamycin exacerbated the disease progression and shortened the survival of ALS mice model, and apoptosis signals were up-regulated compared with control group.
REFERENCES
1.Okado-Matsumoto A., Fridovich I. Amyotrophic lateral sclerosis: a proposed mechanism. Proc Natl Acad Sci USA. 2002. 99:9010–9014.

2.Wang Q., Johnson JL., Agar NY., Agar JN. Protein aggregation and protein instability govern familial amyotrophic lateral sclerosis patient survival. PLoS Biol. 2008. 6:e170.

3.Sreedharan J., Blair IP., Tripathi VB., Hu X., Vance C., Rogelj B, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008. 319:1668–1672.

4.Fornai F., Longone P., Cafaro L., Kastsiuchenka O., Ferrucci M., Manca ML, et al. Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2008. 105:2052–2057.

5.Pasquali L., Longone P., Isidoro C., Ruggieri S., Paparelli A., Fornai F. Autophagy, lithium, and amyotrophic lateral sclerosis. Muscle Nerve. 2009. 40:173–194.

6.Fornai F., Longone P., Ferrucci M., Lenzi P., Isidoro C., Ruggieri S, et al. Autophagy and amyotrophic lateral sclerosis: The multiple roles of lithium. Autophagy. 2008. 4:527–530.

7.Ravikumar B., Vacher C., Berger Z., Davies JE., Luo S., Oroz LG, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004. 36:585–595.

8.Erlich S., Alexandrovich A., Shohami E., Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2007. 26:86–93.

9.Sarkar S., Ravikumar B., Floto RA., Rubinsztein DC. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2009. 16:46–56.

10.Ravikumar B., Berger Z., Vacher C., O’Kane CJ., Rubinsztein DC. Rapamycin pre-treatment protects against apoptosis. Hum Mol Genet. 2006. 15:1209–1216.

11.Pan T., Kondo S., Le W., Jankovic J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain. 2008. 131:1969–1978.

12.Ventruti A., Cuervo AM. Autophagy and neurodegeneration. Curr Neurol Neurosci Rep. 2007. 7:443–451.

13.Ling D., Salvaterra PM. A central role for autophagy in Alzheimer-type neurodegeneration. Autophagy. 2009. 5:738–740.
14.Pan T., Kondo S., Zhu W., Xie W., Jankovic J., Le W. Neuropro-tection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neurobiol Dis. 2008. 32:16–25.

15.Koh SH., Kim Y., Kim HY., Hwang S., Lee CH., Kim SH. Inhibition of glycogen synthase kinase-3 suppresses the onset of symp-toms and disease progression of G93A-SOD1 mouse model of ALS. Exp Neurol. 2007. 205:336–346.

16.Harrison DE., Strong R., Sharp ZD., Nelson JF., Astle CM., Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009. 460:392–395.

17.Zhang X., Li L., Chen S., Yang D., Wang Y., Zhang X, et al. Rapamycin treatment augments motor neuron degeneration in SOD1 (G93A) mouse model of amyotrophic lateral sclerosis. Autophagy. 2011. 7:412–425.
Figure 1.
Results of rotarod test showed that coordinate and strength in G93A transgenic mice was increasingly impaired, however the motor function deficit was alleviated by 15 weeks in G93A mice administrated with rapamycin (A). In the rapamycin administration group, the behavioral function using rotarod test was significantly better in early stage of ALS (Mann-Whitney U test, *p<0.05) (A). However the decline of the rotarod function was more rapid in the rapamycin administration group than the control group after 15 weeks (A). Similarly, the onset of symptoms was significantly delayed by the rapamycin administration (B), whereas the time of rotarod failure (C) and disease end point (D) were shortened in the rapamycin administration group compared with the control group.
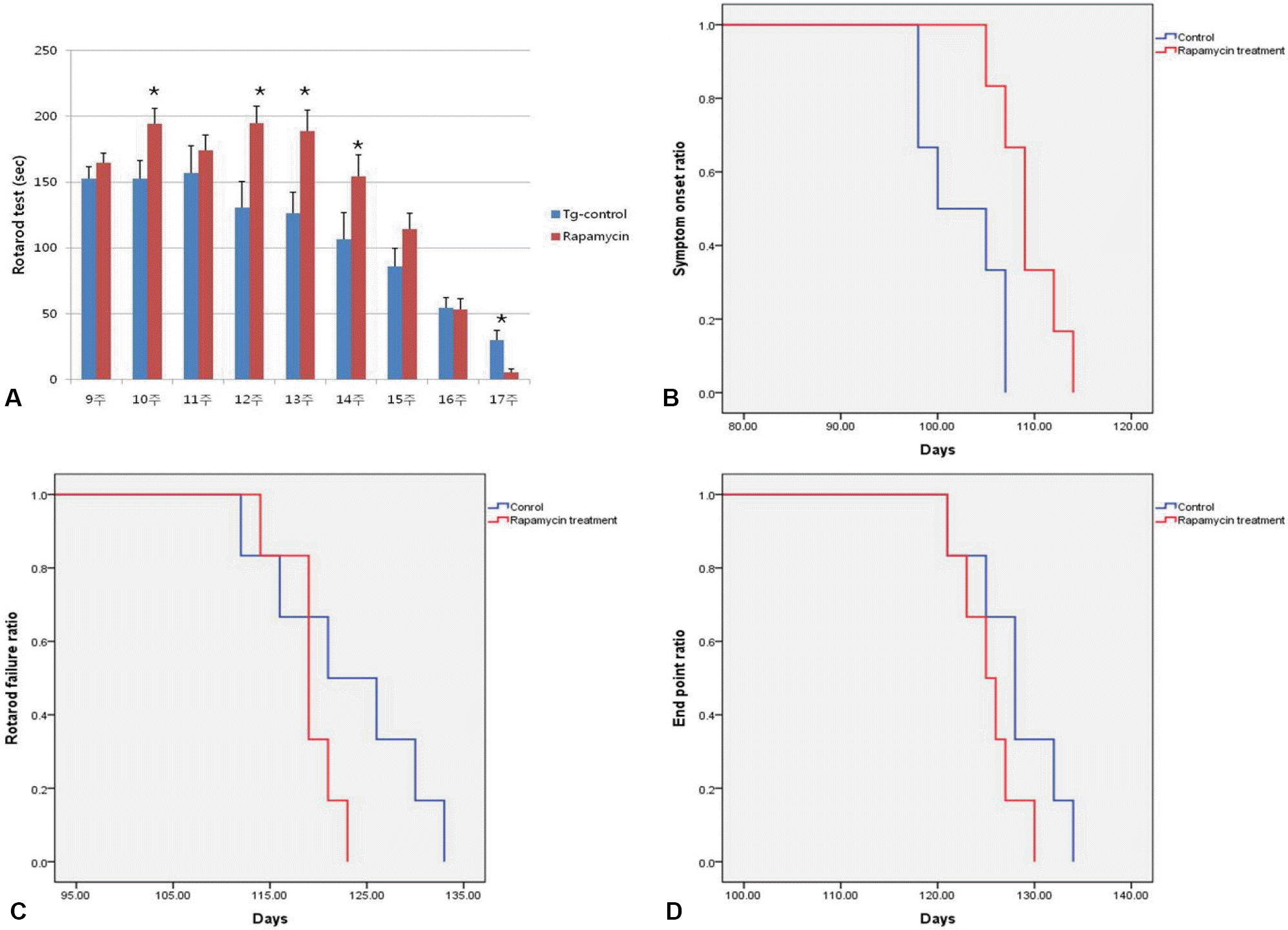
Figure 2.
With the rapamycin administration, the levels of cleaved caspase-8 were increased in the spinal cord of 110-days-old G93A transgenic ALS mice, whereas the level of cleaved caspase-3 was not significant between two groups.
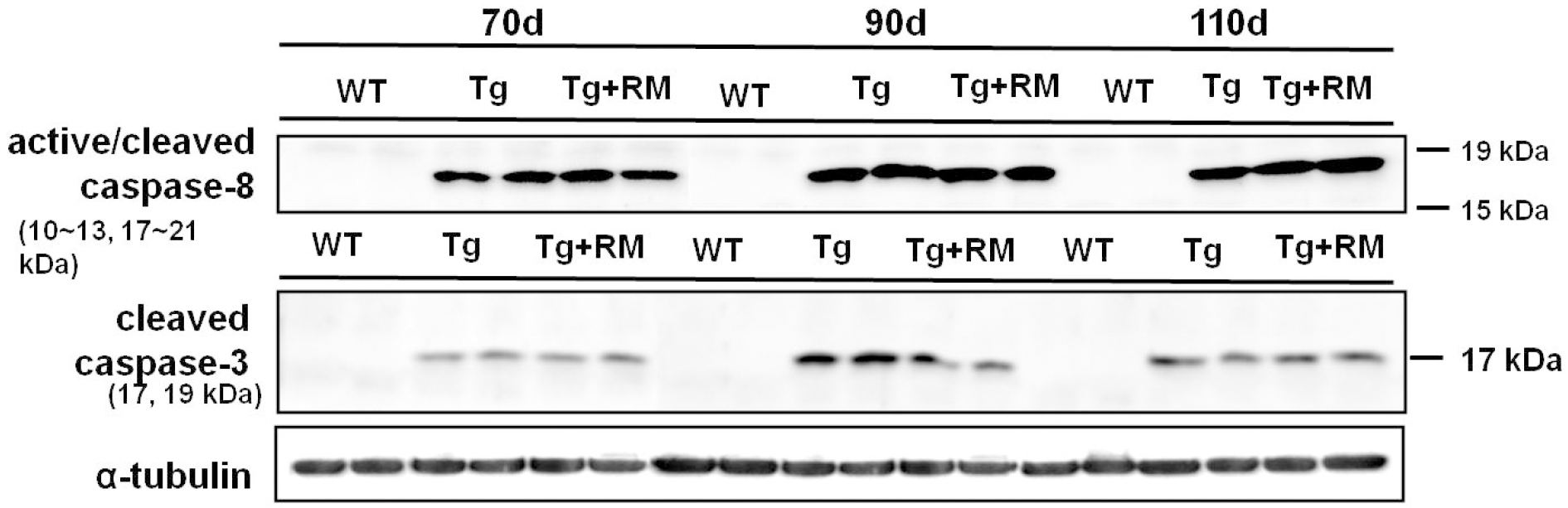
Table 1.
Summary of behavior studies involving symptom onset, rotarod failure, and disease endpoint. The onset of symptoms was significantly delayed in the rapamycin administration group compared with the control group. However the time of rotarod failure and disease end point were shortened in rapamycin treatment group relative to the control group, although the results were not statistically significant




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download