Abstract
Saccades are rapid eye movements that shift the line of sight between successive points of fixation. The cerebellum calibrates saccadic amplitude (dorsal vermis and fastigial nucleus) and the saccadic pulse‐step match (flocculus) for optimal visuo‐ocular motor behavior. Based on electrophysiology and the pharmacological inactivation studies, early activity in one fastigial nucleus could be important for accelerating the eyes at the beginning of a saccade, and the later activity in the other fastigial nucleus could be critical for stopping the eye on target, which is controlled by inhibitory projection from the dorsal vermis. The cerebellum could monitor a corollary discharge of the saccadic command and terminate the eye movement when it is calculated to be on target. The fastigial nucleus and dorsal vermis also participate in the adaptive control of saccadic accuracy.
REFERENCES
1.Pierrot-Deseilligny C., Rivaud S., Gaymard B., Muri RM., Vermersch AI. Cortical control of saccades. Ann Neurol. 1995. 37:557–567.

2.Zee DS., Optican LM., Cook JD., Robinson DA., Engel WK. Slow saccades in spinocerebellar degeneration. Arch Neurol. 1976. 33:243–251.

3.Scudder CA. A new local feedback model of the saccadic burst generator. J Neurophysiol. 1988. 59:1455–1475.

4.Cohen B., Komatsuzaki A., Bender MB. Electrooculographic syndrome in monkeys after pontine reticular formation lesions. Arch Neurol. 1968. 18:78–92.

5.Kaneko CRS. Hypothetical explanation of selective saccadic palsy caused by pontine lesion. Neurology. 1989. 39:994–995.

6.Büttner-Ennever JA., Büttner U. A cell group associated with vertical eye movements in the rostral mesencephalic reticular formation of the monkey. Brain Res. 1978. 151:31–47.

7.Voogd J., Barmack NH. Oculomotor cerebellum. Büttner-Ennever JA, editor. Neuroanatomy of the oculomotor system. Prog Brain Res;2006. 151:p. 231–268.

8.Noda H., Sugita S., Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1990. 302:330–348.

9.May PJ., Hartwich-Young R., Nelson J., Sparks DL., Porter JD. Cerebellotectal pathways in the macaque: implications for col-licular generation of saccades. Neuroscience. 1990. 36:305–324.

10.Ohtsuka K., Noda H. Saccadic burst neurons in the oculomotor region of the fastigial neurons in macaque monkeys. J Neurophysiol. 1992. 65:1422–1434.
11.Fuchs AF., Robinson FR., Straube A. Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge patterns. J Neurophysiol. 1993. 70:1723–1740.
12.Robinson FR., Straube A., Fuchs AF. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol. 1993. 70:1741–1758.

13.Ohtsuka K., Noda H. Discharge properties of Purkinje cells in the oculomotor vermis during visually guided saccades in the macaque monkey. J Neurophysiol. 1995. 74:1828–1840.

14.Noda H., Fujikado T. Topography of the oculomotor area of the cerebellar vermis in macaques as determined by microstimulation. J Neurophysiol. 1987. 58:359–378.

15.Ron S., Robinson DA. Eye movements evoked by cerebellar stimulation in the alert monkey. J Neurophysiol. 1973. 1004–1022.

16.Sato H., Noda H. Saccadic dysmetria induced by transient functional decortication of the cerebellar vermis. Exp Brain Res. 1992. 88:455–458.

17.Büttner-Ennever JA., Horn AK. Pathways from cell groups of the paramedian tracts to the floccular region. Ann N Y Acad Sci. 1996. 781:532–540.
18.Desmurget M., Pelisson D., Grethe JS., Alexander GE., Urquizar C., Prablanc C, et al. Functional adaptation of reactive saccades in humans. A PET study. Exp Brain Res. 2000. 132:243–259.

19.Robinson FR., Fuchs AF., Noto CT. Cerebellar influences on saccade plasticity. Ann N Y Acad Sci. 2002. 956:155–163.

20.Scudder CA., McGee DM. Adaptive modification of saccade size produces correlated changes in the discharges of fastigial nucleus neurons. J Neurophysiol. 2003. 90:1011–1026.
Figure 1.
The major structures for saccades. The cerebellum receives saccadic commands, which are relayed by NRTP from the frontal eye fields and superior colliculus. FEF; frontal eye fields, DLPC; dorsolateral prefrontal cortex, SEF; supplementary eye fields, PEF; parietal eye fields, PPC; posterior parietal cortex, IML; intramedullary lamina of thalamus, SNpr; substantia nigra pars reticularis, STN; subthalamic nucleus, NRTP; nucleus reticularis tegmenti pontis.
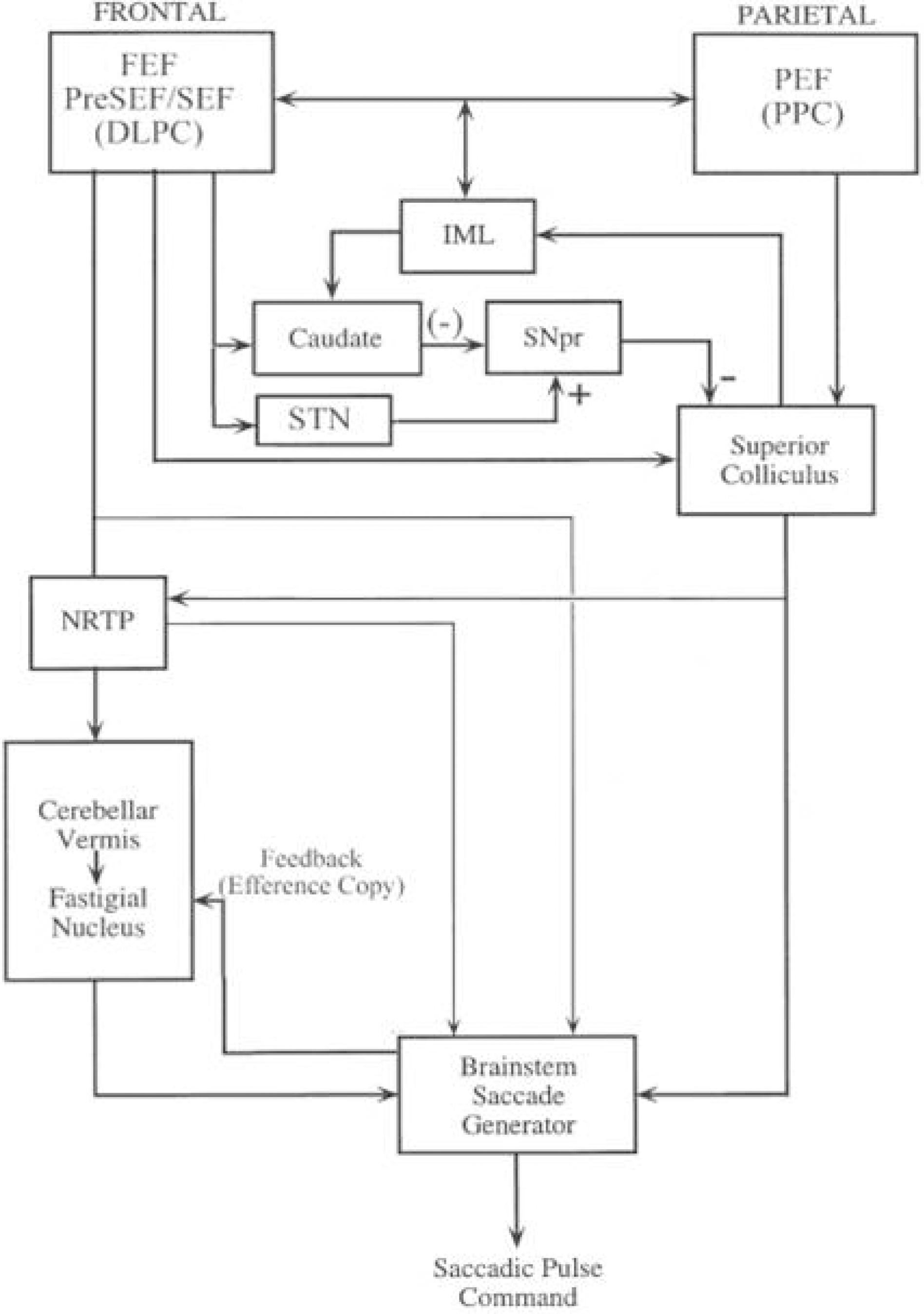
Figure 3.
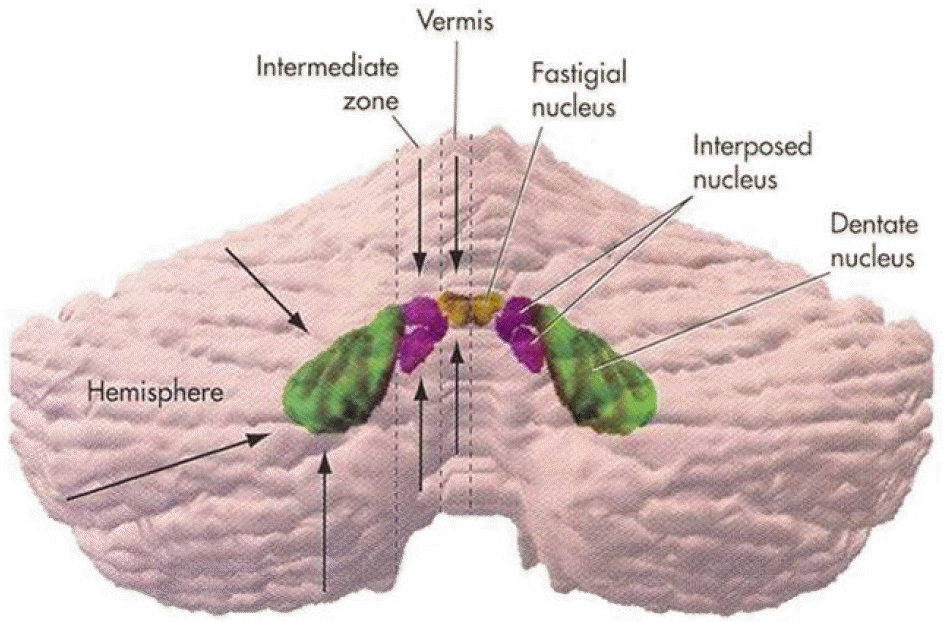
Lobules of the cerebellum. The “ocular motor vermis” consists of lobules VI and VII (part of the declive, folium, tuber, and pyramids).
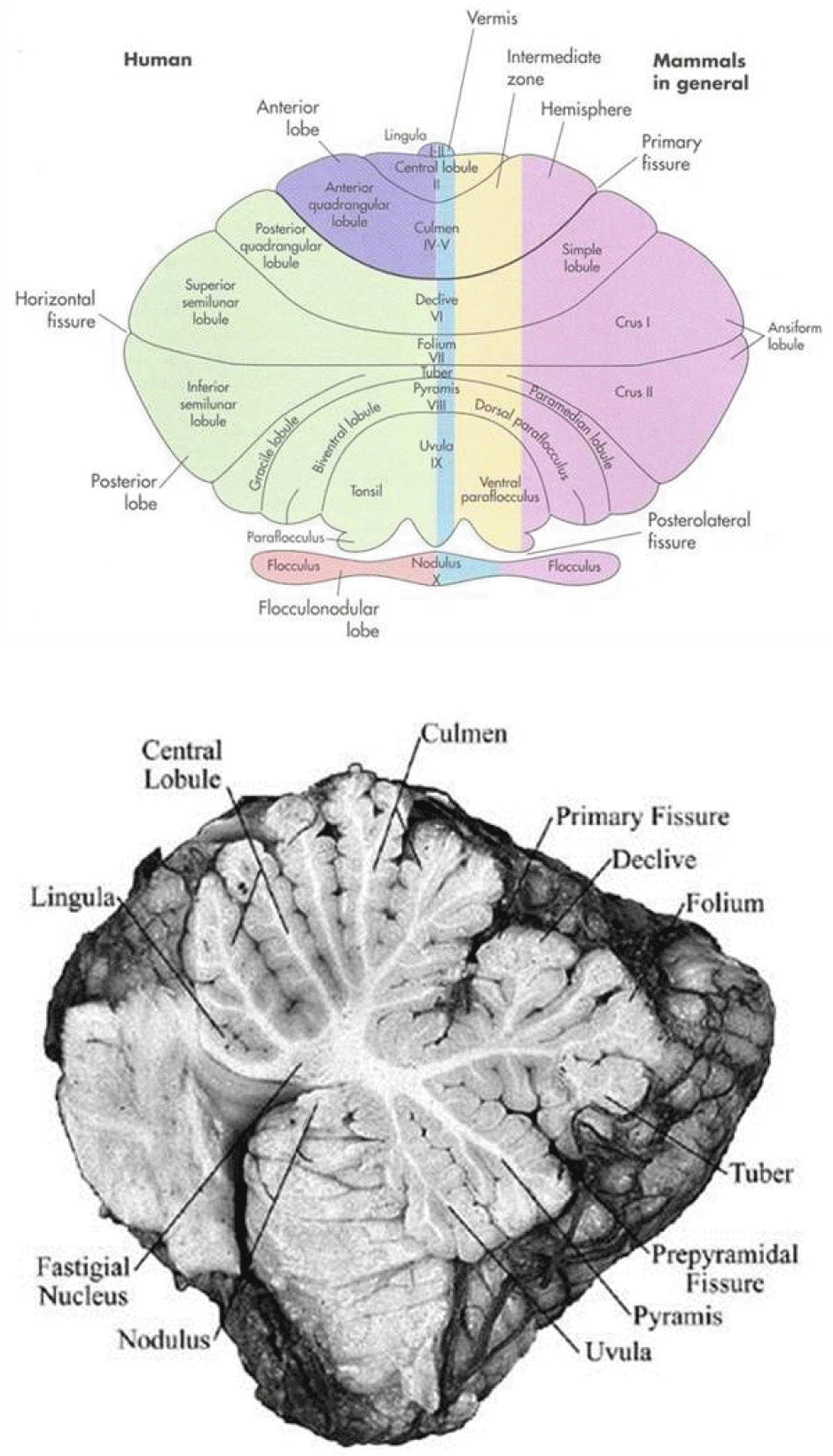
Figure 4.
The procedures for the adaptation paradigm. In each frame, the stimulus is represented by a grey square and the current eye position by a cross. (1) The subject fixates the stimulus. (2) The stimulus is extinguished and simultaneously reappears 30° away from the fixation point. (3) While the eyes are moving to the new location, the stimulus is diplaced 8° backward. The displacement of target to one side leads directly to the other side in a continuous left/right alternation. The subjects perform 200 saccades while the target steps backward during the primary saccade.
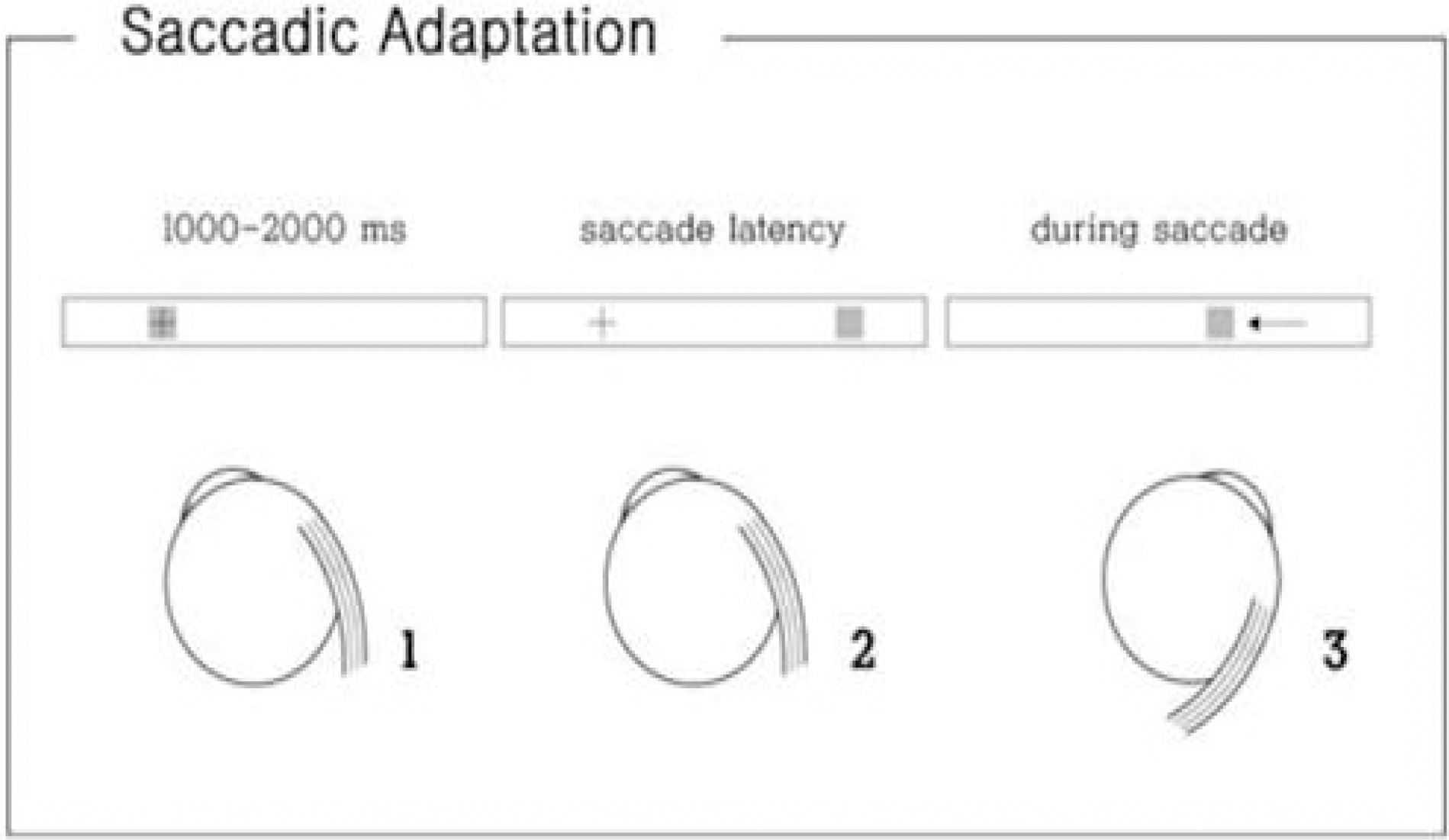
Figure 5.
Adaptive gain change of ipsi‐ and contralesional saccades in a patient with right cerebellar infarction. The saccadic gain adaptation is reduced only ipsilesionally.
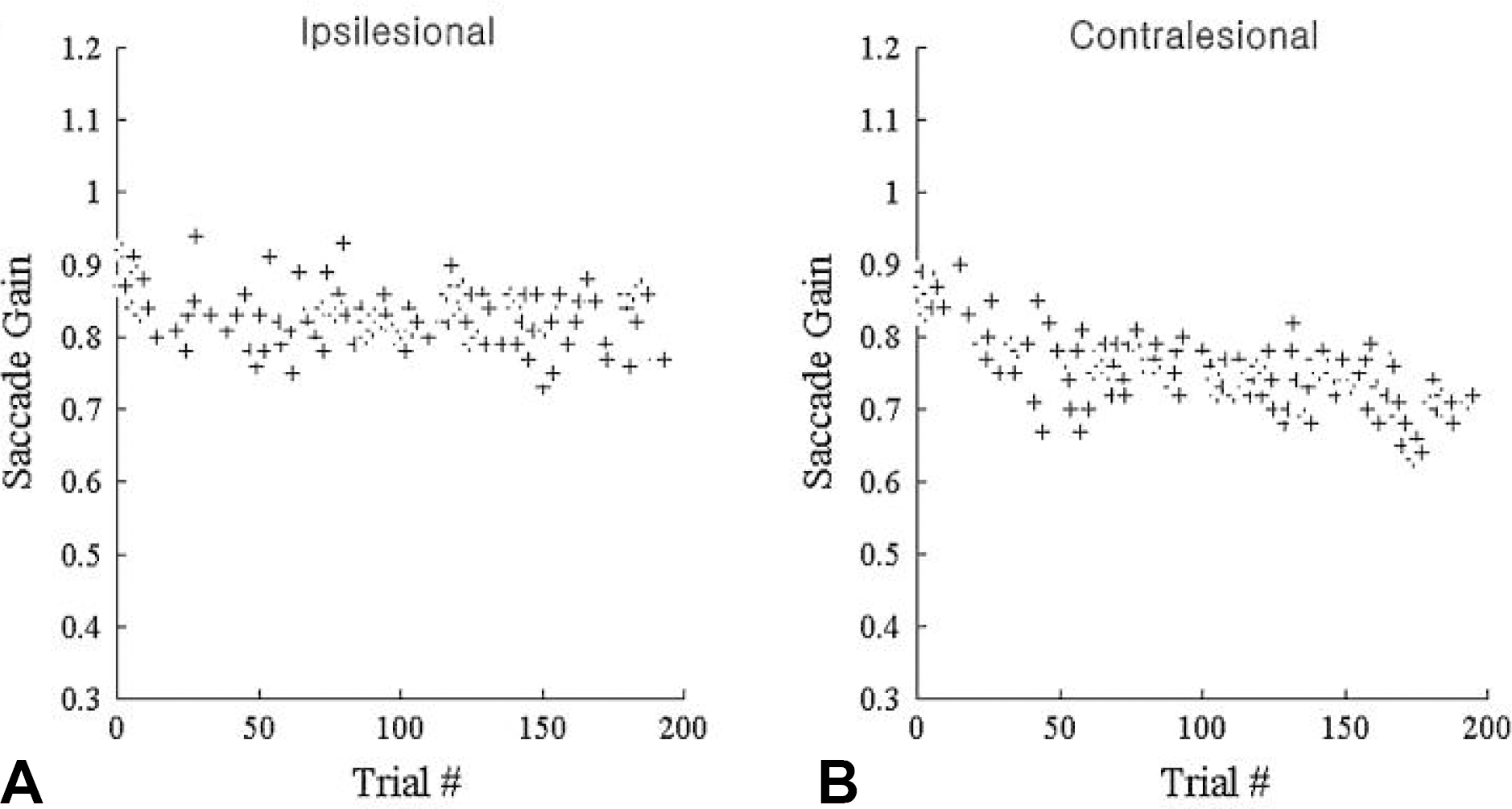
Table 1.
Saccadic abnormalities with lesions affecting the dorsal vermis and fastigial nuelcus




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download