Abstract
Objective
The risk factors of Clostridium difficile-associated diarrhea (CDAD) are well known in medical part. However, there have been a few studies of CDAD about neurosurgical patients. The aim of this study was to investigate clinical characteristics and risk factors of neurosurgical patients with CDAD.
Methods
We retrospectively reviewed the record of eighty-five patients with CDAD between January 2007 and December 2010. They made a diagnosis that used a toxin assay, stool culture and sigmoidoscopy or colonoscopy. We analyzed the association factor such as age, gender, operation, enteral feeding, length of Intensive Care Unit stay, prophylactic antibiotics, duration and number of antibiotics, types of antibiotics, diarrhea onset time, toxin assay, serum albumin, C-reactive protein, pseudomembranous colitis, recurrence rates between the neurologically impaired group and well-active group.
Results
Of 15 parameters, 6 parameters were significantly associated with neurologically impaired group in univariate analysis. The enteral feeding and bed ridden state (p<0.001) frequently had practiced and the intensive care unit stay had longer (p=0.001) and the diarrhea onset time (p=0.034) from the last antibiotics use administered prior to development of the CDAD was lesser. The pseudomembranous colitis and CDAD recurrence had more appeared in impaired group (p<0.001). On multivariate analysis, longer intensive care unit stay (p=0.024) and increasing cumulative days of antibiotic administration (p=0.007) correlated with pseudomembranous colitis.
Figures and Tables
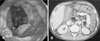 | FIGURE 1Descending colon covered with yellowish pseudo-membrane on sigmoidoscopy (A) and CT scan of the abdomen (B) showing gross thickening of the large bowel wall and obliteration of the lumen. |
TABLE 1
Comparisons of clinical characteristics between neurologically impaired and well active group

References
1. Barbut F, Petit JC. [Epidemiology, risk factors and prevention of Clostridium difficile nosocomial infections]. Pathol Biol (Paris). 2000; 48:745–755.
3. Bulstrode NW, Bradbury AW, Barrett S, Stansby G, Mansfield AO, Nicolaides AN, et al. Clostridium difficile colitis after aortic surgery. Eur J Vasc Endovasc Surg. 1997; 14:217–220.

4. Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010; 31:431–455.

5. Crabtree T, Aitchison D, Meyers BF, Tymkew H, Smith JR, Guthrie TJ, et al. Clostridium difficile in cardiac surgery: risk factors and impact on postoperative outcome. Ann Thorac Surg. 2007; 83:1396–1402.

6. Frost F, Hurley JS, Petersen HV, Casciano RN. Estimated incidence of Clostridium difficile infection. Emerg Infect Dis. 1999; 5:303–304.

7. Gerding DN, Johnson S. Harrison's principles of internal medicine. ed 16. McGraw-Hill;2005. p. 760–762.
8. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995; 16:459–477.

9. Hashimoto M, Sugawara Y, Tamura S, Kaneko J, Matsui Y, Togashi J, et al. Clostridium difficile-associated diarrhea after living donor liver transplantation. World J Gastroenterol. 2007; 13:2072–2076.

10. Hirschhorn LR, Trnka Y, Onderdonk A, Lee ML, Platt R. Epidemiology of community-acquired Clostridium difficile-associated diarrhea. J Infect Dis. 1994; 169:127–133.

11. Hookman P, Barkin JS. Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol. 2009; 15:1554–1580.
12. Johnson S, Kent SA, O'Leary KJ, Merrigan MM, Sambol SP, Peterson LR, et al. Fatal pseudomembranous colitis associated with a variant clostridium difficile strain not detected by toxin A immunoassay. Ann Intern Med. 2001; 135:434–438.

13. Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001; 357:189–193.

14. Larson HE, Price AB, Honour P, Borriello SP. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet. 1978; 1:1063–1066.
15. Lee KS, Shin WG, Jang MK, Kim HS, Kim HS, Park CJ, et al. Who are susceptible to pseudomembranous colitis among patients with presumed antibiotic-associated diarrhea? Dis Colon Rectum. 2006; 49:1552–1558.

16. Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005; 353:2442–2449.
17. Lyerly DM, Krivan HC, Wilkins TD. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988; 1:1–18.

18. McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989; 320:204–210.

19. Morris AM, Jobe BA, Stoney M, Sheppard BC, Deveney CW, Deveney KE. Clostridium difficile colitis: an increasingly aggressive iatrogenic disease? Arch Surg. 2002; 137:1096–1100.
20. Oldfield EC 3rd. Clostridium difficile-associated diarrhea: risk factors, diagnostic methods, and treatment. Rev Gastroenterol Disord. 2004; 4:186–195.
21. Owens RC Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008; 46:Suppl 1. S19–S31.
22. Park BS, Kim JH, Seo HI, Kim HS, Kim DH, Cho HJ, et al. Pseudomembranous colitis after gastrointestinal operation. J Korean Surg Soc. 2009; 77:106–112.

23. Pépin J, Valiquette L, Alary ME, Villemure P, Pelletier A, Forget K, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004; 171:466–472.
24. Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005; 173:1037–1042.
26. Shim JK, Johnson S, Samore MH, Bliss DZ, Gerding DN. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet. 1998; 351:633–636.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download