Abstract
Objective
Energy failure and concurrent electrical silence are common features of oxygen-glucose deprivation (OGD) in the brain. Hippocampal slice has been used extensively to study electrophysiological alterations. Orthodromic extracellular field potential recording has been most widely chosen for those studies however there were few with antidromic recording. The goal of this study is to clarify which types of recordings is better for the evaluation of extent of ischecmic insults.
Methods
Rat hippocampal slices were made for the orthodromically and antidromically evoked filed potential recording. Before, during and after 6 to 11 minutes of experimental OGD, the authors measured population spike amplitude and slope of field excitatory postsynaptic potential (fEPSP).
Results
A dramatic reduction of amplitude and total disappearance of orthodromic population spike (oPS) noted 1.1 +/- 0.2 min after OGD onset. On the contrary antidromic population spike (aPS) was not affected at the beginning. It slowly and gradually diminished and finally disappeared 6.6 +/- 0.2 min after OGD onset. A transient recovery of oPS, so called hypoxic injury potentials (HIP) briefly occurred just before the total dissappearance of aPS and the both signals disappeared simultaneously. Incomplete recovery due to irreversible damage began 7 min after OGD onset. There was no recovery 10 min after OGD, 7 min after oPS loss and 2 min after aPS loss. The OGD experiments with various neuroprotective agents (MK 801, AP-5, lidocaine, CNQX, adenosine) lasted for longer than 2 min after aPS disappearance sensitively showed their efficacy.
Figures and Tables
FIGURE 1
Schematic drawing showing the experimental setup for the electrophysiological recordings from CA1 hippocampus.
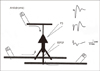
FIGURE 2
A: Graph showing a representative tracing of ortho-(open circle, oPS) and anti-dromic (filled circle, aPS) population spikes before, during and after 8 minutes of oxygen-glucose deprivation. The depression of oPS noted first and a transient recovery was followed immediately before the complete dissappearance of aPS. B: Bar graph showing the relationship between the total duration of ischemia and the sequential recovery of oPS.

FIGURE 3
A: Scatter graph showing the relationship between the duration of hypoxic injury potential and the recovery of aPS. The experimental conditions that limit HIP duration to less than 1 minutes elicited poor recovery. B: Scatter graph showing the relationship between the duration of ischemia after the initial depression of oPS and the recovery of aPS. C: Scatter graph showing the relationship between the duration of ischemia lasting later the depression of aPS and the recovery of aPS.

FIGURE 4
Scatter graphs showing the effect of various neuro-protective agents and -modulators. The control data in these graphs are the same as those shown in Fig. 3C. The data point shown on the right side of two vertical dotted line and on the upper part of horizontal line means that it has some neuroprotective potentials.

TABLE 1
Result of two different recordings obtained from the same 9 min of ischemia experiments. All the agents shown to be effective in oPS recordings (20 uM MK-801, 40 uM Lidocaine) are also effective in aPS recording. In addition, those without effect in oPS recording (20 uM Lidocaine and 10 uM CNQX) are much clearer in aPS recording

References
1. Andersen P, Eccles JC, Loyning Y. Pathway of postsynaptic inhibition in the hippocampus. J Neurophysiol. 1964; 27:608–619.

2. Andersen P, Silfvenius H, Sundberg SH, Sveen O. A comparison of distal and proximal dendritic synapses on CAi pyramids in guinea-pig hippocampal slices in vitro. J Physiol. 1980; 307:273–299.
3. Andersen P, Silfvenius H, Sundberg SH, Sveen O, Wigström H. Functional characteristics of unmyelinated fibres in the hippocampal cortex. Brain Res. 1978; 144:11–18.

4. Balestrino M, Aitken PG, Somjen GG. The effects of moderate changes of extracellular K+ and Ca2+ on synaptic and neural function in the CA1 region of the hippocampal slice. Brain Res. 1986; 377:229–239.

5. Cherubini E, Rovira C, Ben-Ari Y, Padjen A. Simultaneous recording of somatic and dendritic field potentials and combined microiontophoresis in the rat Ammon's horn in situ: effects of GABA and acetylcholine. Neurosci Lett. 1982; 31:19–24.

6. Corbett D, Nurse S. The problem of assessing effective neuroprotection in experimental cerebral ischemia. Prog Neurobiol. 1998; 54:531–548.

7. Danscher G, Shipley MT, Andersen P. Persistent function of mossy fibre synapses after metal chelation with DEDTC (Antabuse). Brain Res. 1975; 85:522–526.

8. de Mendonça A, Ribeiro JA. Adenosine inhibits the NMDA receptor-mediated excitatory postsynaptic potential in the hippocampus. Brain Res. 1993; 606:351–356.

9. de Mendonça A, Sebastião AM, Ribeiro JA. Inhibition of NMDA receptor-mediated currents in isolated rat hippocampal neurones by adenosine A1 receptor activation. Neuroreport. 1995; 6:1097–1100.

10. Doolette DJ, Kerr DI. Hyperexcitability in CA1 of the rat hippocampal slice following hypoxia or adenosine. Brain Res. 1995; 677:127–137.

11. Fairchild MD, Parsons JE, Wasterlain CG, Rinaldi PC, Wallis RA. A hypoxic injury potential in the hippocampal slice. Brain Res. 1988; 453:357–361.

12. Fowler JC. Adenosine antagonists alter the synaptic response to in vitro ischemia in the rat hippocampus. Brain Res. 1990; 509:331–334.

13. Jester JM, Campbell LW, Sejnowski TJ. Associative EPSP--spike potentiation induced by pairing orthodromic and antidromic stimulation in rat hippocampal slices. J Physiol. 1995; 484:689–705.

14. Klishin A, Lozovaya N, Krishtal O. A1 adenosine receptors differentially regulate the N-methyl-D-aspartate and non-N-methyl-D-aspartate receptor-mediated components of hippocampal excitatory postsynaptic current in a Ca2+/Mg(2+)-dependent manner. Neuroscience. 1995; 65:947–953.

15. McCullers DL, Sullivan PG, Scheff SW, Herman JP. Mifepristone protects CA1 hippocampal neurons following traumatic brain injury in rat. Neuroscience. 2002; 109:219–230.

16. Miyashita K, Nakajima T, Ishikawa A, Miyatake T. An adenosine uptake blocker, propentofylline, reduces glutamate release in gerbil hippocampus following transient forebrain ischemia. Neurochem Res. 1992; 17:147–150.

17. Niiyama S, Tanaka E, Yamamoto S, Yasumoto S, Kano T, Higashi H. Bupivacaine, but not tetracaine, protects against the in vitro ischemic insult of rat hippocampal CA1 neurons. Neurosci Res. 2002; 42:231–241.

18. Oliveira IJ, Molz S, Souza DO, Tasca CI. Neuroprotective effect of GMP in hippocampal slices submitted to an in vitro model of ischemia. Cell Mol Neurobiol. 2002; 22:335–344.
19. Preston E, Webster J. Spectrophotometric measurement of experimental brain injury. J Neurosci Methods. 2000; 94:187–192.

20. Rekling JC. Neuroprotective effects of anticonvulsants in rat hippocampal slice cultures exposed to oxygen/glucose deprivation. Neurosci Lett. 2003; 335:167–170.

21. Schmidt-Kastner R, Truettner J, Lin B, Zhao W, Saul I, Busto R, Ginsberg MD. Transient changes of brain-derived neurotrophic factor (BDNF) mRNA expression in hippocampus during moderate ischemia induced by chronic bilateral common carotid artery occlusions in the rat. Brain Res Mol Brain Res. 15; 92(1-2):157–166.

22. Sebastião AM, de Mendonca A, Moreira T, Ribeiro JA. Activation of synaptic NMDA receptors by action potential-dependent release of transmitter during hypoxia impairs recovery of synaptic transmission on reoxygenation. J Neurosci. 2001; 21:8564–8571.

23. Shinskaia NE. [Role of cAMP in regulating hippocampal neuronal reactivity in vitro]. Biull Eksp Biol Med. 1982; 94:3–5.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download