Abstract
Objective
Potassium channels are ubiquitously expressed in all organisms and constitute the most diverse class of ion channels. Their action is regulated by various factors and is involved in neuronal excitability. 4-aminopyridine (4-AP) is a voltage-sensitive K+ channel inhibitor used in studies of experimental neuronal excitation and in the treatment of demyelinating diseases. It is also known as having a strong convulsant activity on hippocampal slices. We investigated the effect of several K+ channel antagonists on evoked potentials during normoxia and hypoxia.
Methods
Field excitatory postsynaptic potentials (fEPSPs), orthodromic (oPSs) and antidromic population spikes (aPSs) were recorded simultaneously in the cornus ammoni1 (CA1) area of rat hippocampal slices. fEPSP/Spike (E-S) ratio was calculated during the experiments to observe sequential changes of pre- and postsynaptic neuronal excitability, especially epileptiform activities. 4-AP, tetraethylammonium (TEA), dequalinium dichloride, paxilline, tertiapin-Q, glibenclamide and barium chloride were tested.
Results
Most of the tested K+ channel blockers showed increase of mean amplitudes in oPS, fEPSP and aPS compared to control slices. 4-AP showed most strong increase in oPS (188.5-2.1%). During the early period of hypoxia, epileptiform activities were observed more prominently in 4-AP treated slices, which were not produced in other K+ channel blockers. Those epileptiform activities were accompanied by E-S potentiation (mean 426.6%). Common antiepileptic drugs-valproic acid, phenytoin and carbamazepine-effectively controlled epileptiform activities induced by 4-AP during hypoxia (p=0.0035).
Conclusion
Our experimental results thus show that 1) potassium channels significantly increase evoked field potentials, 2) epileptiform activities are increased in 4-AP treated slices during hypoxia and they are attributed to E-S potentiation and 3) 4-AP induced epileptiform activities during hypoxia are effectively controlled by antiepileptic drugs.
Figures and Tables
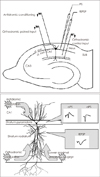 | FIGURE 1Upper: Schematic diagrams representing locations of stimulating and recording sites in CA1 hippocampal region. Lower: Three stimulating sites (orthodromic control input, paired input and antidromic conditioning) and two recording sites (PS, fEPSP). CA: cornus ammoni, PS: population spike, fEPSP: field ex-citatory postsynaptic potentials. |
 | FIGURE 2Sample tracing of evoked potentials treated by 4-aminopyridine (4-AP). orthodromic (oPS) are significantly increased after superfusing 4-AP (compare a and b), while fEPSP and antidromic population spike (aPS) does not change during the time course. fEPSP: field excitatory postsynaptic potentials. |
 | FIGURE 3Mean amplitudes of each evoked potentials after superfusion of various potassium channel blockers. A: oPS: All potassium channel blockers shows significantly increased amplitudes of oPS compared to control slices. 4-AP shows largest increase (188.5±2.1%) in oPS. B: fEPSP: Kv blockers show more increase fEPSP than other antagonists and TEA shows most prominent effect (141.8±1.5%). C: aPS: Compared to oPS and fEPSP, aPS shows rather small changes. Barium chloride has most prominent effect (140±3.7%). Asterisk(*) indicates p<0.05 of mean amplitudes between control and test slices (Student's t test for unpaired observations). oPS: orthodromic, fEPSP: field excitatory postsynaptic potentials, aPS: antidromic population spike, Kv: voltage dependent potassium channel, 4-AP: 4-aminopyridine, TEA: tetraethylammonium. |
 | FIGURE 4Sequential changes of evoked potentials during hypoxia with A1 adenosine antagonist. A: During hypoxia, both oPS and fEPSP rapidly depresses and recover after reoxygenation and show posthypoxic excitation. B: After A1 receptor blocking, depressed fEPSP and oPS are largely attenuated. Note the differences of recovered amplitudes of fEPSP and oPS. (a): E-S poten-tiation and (b): E-S depression. C: In slices superfused with 4-AP, E-S potentiation (c) is appeared during hypoxia. D: Similar result with more attenuated fEPSP and oPS [with E-S potentiation(d)] during hypoxia in slices with DPCPX. oPS: orthodromic, fEPSP: field excitatory postsynaptic potentials, E-S: EPSP-Spike, 4-AP: 4-aminopyridine. |
 | FIGURE 5Increase of epileptiform activities (additional PS) during hypoxia in slices with 4-AP. Normal tracing (a) and typical samples showing epileptiform activities appearing after superfusiing 4-AP (b) and exaggerated by additional hypoxia (c). Sequential changes of E-S ratio shows prominent E-S potentiation during hypoxia (filled squares). PS: population spike, 4-AP: 4-aminopyridine, E-S: EPSP-Spike. |
 | FIGURE 6Comparing E-S ratio between hypoxia (A) and 4-AP with hypoxia (B). Sample tracing and mean shows that E-S depression during hypoxia (A) while E-S potentiation in slices with 4-AP and 4-AP with hypoxia (B). E-S: EPSP-Spike, 4-AP: 4-aminopyridine. |
References
1. Avoli M, Barbarosie M, Lücke A, Nagao T, Lopantsev V, Köhling R. Synchronous GABA-mediated potentials and epileptiform discharges in the rat limbic system in vitro. J Neurosci. 1996; 16:3912–3924.
2. Bernard C, Pickering J, Wheal HV. Reversal of excitatory postsynaptic potential/spike potentiation in the CA1 area of the rat hippocampus. Neuroscience. 1998; 86:431–436.

3. Bernard C, Wheal HV. Expression of EPSP/spike potentiation following low frequency and tetanic stimulation in the CA1 area of the rat hippocampus. J Neurosci. 1995; 15:6542–6551.

4. Bever CT Jr, Young D, Anderson PA, Krumholz A, Conway K, Leslie J, et al. The effects of 4-aminopyridine in multiple sclerosis patients: results of a randomized, placebo-controlled, double-blind, concentration-controlled, crossover trial. Neurology. 1994; 44:1054–1059.

5. Blight AR, Gruner JA. Augmentation by 4-aminopyridine of vestibulospinal free fall responses in chronic spinal-injured cats. J Neurol Sci. 1987; 82:145–159.

6. Daoudal G, Hanada Y, Debanne D. Bidirectional plasticity of excitatory postsynaptic potential (EPSP)-spike coupling in CA1 hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2002; 99:14512–14517.

7. de Mendonça A, Sebastião AM, Ribeiro JA. Adenosine: does it have a neuroprotective role after all? Brain Res Brain Res Rev. 2000; 33:258–274.

8. Dodson PD, Forsythe ID. Presynaptic K+ channels: electrifying regulators of synaptic terminal excitability. Trends Neurosci. 2004; 27:210–217.

9. Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001; 24:31–55.

10. Fowler JC. Adenosine antagonists delay hypoxia-induced depression of neuronal activity in hippocampal brain slice. Brain Res. 1989; 490:378–384.

11. Fueta Y, Avoli M. Effects of antiepileptic drugs on 4-aminopyridine-induced epileptiform activity in young and adult rat hippocampus. Epilepsy Res. 1992; 12:207–215.

12. Fujii S, Kuroda Y, Ito K, Kaneko K, Kato H. Effects of adenosine receptors on the synaptic and EPSP-spike components of long-term potentiation and depotentiation in the guinea-pig hippocampus. J Physiol. 1999; 521 Pt 2:451–466.

13. Fujiwara N, Higashi H, Shimoji K, Yoshimura M. Effects of hypoxia on rat hippocampal neurones in vitro. J Physiol. 1987; 384:131–151.

14. Godukhin O, Savin A, Kalemenev S, Levin S. Neuronal hyperexcitability induced by repeated brief episodes of hypoxia in rat hippocampal slices: involvement of ionotropic glutamate receptors and L-type Ca(2+) channels. Neuropharmacology. 2002; 42:459–466.

15. Griesemer D, Zawar C, Neumcke B. Cell-type specific depression of neuronal excitability in rat hippocampus by activation of ATP-sensitive potassium channels. Eur Biophys J. 2002; 31:467–477.

16. Gu Y, Ge SY, Ruan DY. Effect of 4-aminopyridine on synaptic transmission in rat hippocampal slices. Brain Res. 2004; 1006:225–232.

17. Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, et al. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev. 2003; 55:583–586.

18. Hayes KC, Potter PJ, Hsieh JT, Katz MA, Blight AR, Cohen R. Pharmacokinetics and safety of multiple oral doses of sustained-release 4-aminopyridine (Fampridine-SR) in subjects with chronic, incomplete spinal cord injury. Arch Phys Med Rehabil. 2004; 85:29–34.
19. Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997; 387:869–875.

20. Jan LY, Jan YN. Voltage-gated and inwardly rectifying potassium channels. J Physiol. 1997; 505:267–282.

21. Kirsch GE, Shieh CC, Drewe JA, Vener DF, Brown AM. Segmental exchanges define 4-aminopyridine binding and the inner mouth of K+ pores. Neuron. 1993; 11:503–512.

22. Magee JC. Dendritic integration of excitatory synaptic input. Nat Rev Neurosci. 2000; 1:181–190.

23. Magee JC, Johnston D. Synaptic activation of voltage-gated channels in the dendrites of hippocampal pyramidal neurons. Science. 1995; 268:301–304.

24. Mattia D, Nagao T, Rogawski MA, Avoli M. Potassium channel activators counteract anoxic hyperexcitability but not 4-aminopyridine-induced epileptiform activity in the rat hippocampal slice. Neuropharmacology. 1994; 33:1515–1522.

25. Miller C. An overview of the potassium channel family. Genome Biol. 2000; 1:REVIEWS0004.
26. Nieber K. Hypoxia and neuronal function under in vitro conditions. Pharmacol Ther. 1999; 82:71–86.

27. O'Kane EM, Stone TW. Barium, glibenclamide and CGS21680 prevent adenosine A1 receptor changes of ES coupling and spike threshold. Neurosignals. 2004; 13:318–324.
28. Peña F, Bargas J, Tapia R. Paired pulse facilitation is turned into paired pulse depression in hippocampal slices after epilepsy induced by 4-aminopyridine in vivo. Neuropharmacology. 2002; 42:807–812.

29. Peña F, Tapia R. Relationships among seizures, extracellular amino acid changes, and neurodegeneration induced by 4-aminopyridine in rat hippocampus: a microdialysis and electroencephalographic study. J Neurochem. 1999; 72:2006–2014.

30. Pongs O. Voltage-gated potassium channels: from hyperexcitability to excitement. FEBS Lett. 1999; 452:31–35.

31. Rader RK, Lanthorn TH. Experimental ischemia induces a persistent depolarization blocked by decreased calcium and NMDA antagonists. Neurosci Lett. 1989; 99:125–130.

32. Schechter LE. The potassium channel blockers 4-aminopyridine and tetraethylammonium increase the spontaneous basal release of [3H]5-hydroxytryptamine in rat hippocampal slices. J Pharmacol Exp Ther. 1997; 282:262–270.
33. Schubert P, Rudolphi KA, Fredholm BB, Nakamura Y. Modulation of nerve and glial function by adenosine--role in the development of ischemic damage. Int J Biochem. 1994; 26:1227–1236.

34. Semyanov A, Godukhin O. Epileptiform activity and EPSP-spike potentiation induced in rat hippocampal CA1 slices by repeated high-K(+): involvement of ionotropic glutamate receptors and Ca (2+)/calmodulin-dependent protein kinase II. Neuropharmacology. 2001; 40:203–211.
35. Smith KJ, Felts PA, John GR. Effects of 4-aminopyridine on demyelinated axons, synapses and muscle tension. Brain. 2000; 123:171–184.

36. Solari A, Uitdehaag B, Giuliani G, Pucci E, Taus C. Aminopyridines for symptomatic treatment in multiple sclerosis. Cochrane Database Syst Rev. 2002; CD001330.

37. Sperlágh B, Zsilla G, Vizi ES. K(ATP) channel blockers selectively interact with A(1)-adenosine receptor mediated modulation of acetylcholine release in the rat hippocampus. Brain Res. 2001; 889:63–70.

38. Takigawa T, Alzheimer C. G protein-activated inwardly rectifying K+ (GIRK) currents in dendrites of rat neocortical pyramidal cells. J Physiol. 1999; 517:385–390.
39. Taube JS, Schwartzkroin PA. Mechanisms of long-term potentiation: a current-source density analysis. J Neurosci. 1988; 8:1645–1655.

40. Wolfe DL, Hayes KC, Hsieh JT, Potter PJ. Effects of 4-aminopyridine on motor evoked potentials in patients with spinal cord injury: a double-blinded, placebo-controlled crossover trial. J Neurotrauma. 2001; 18:757–771.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download