Abstract
Interhemispheric subdural hematoma (ISH) presenting with falx syndrome is a rare occurrence. We present a case of ISH presenting with falx syndrome with visual disturbances. A 66-year-old woman reported headache as a result of a fall. Initial nonenhanced computed tomography (CT) showed a left frontal ISH. Two days later, she was noted to have right homonymous hemianopsia and marked right hemiparesis, especially in the right lower extremity. Follow up CT demonstrated an increase in the size of the frontal ISH and a newly developed occipital ISH. On thirteen day after the trauma, she was treated with mannitol and dexamethasone. Twelve hours after the start of the mannitol and dexamethasone therapy, the patient showed marked improvement of the neurologic deficit. Five months after the trauma, she showed no neurological deficits.
Isolated interhemispheric subdural hematoma (ISH) represents one of the rarest forms of posttraumatic intracranial hemorrhage. ISH was first described at autopsy by Aring and Evans in 19401) and was first recognized in a living person by Jacobson in 1955. Although a subdural hematoma usually occurs over the convexity of a hemisphere, it occurs occasionally in the interhemispheric fissure. ISH usually occurs after head trauma, and its spontaneous or nontraumatic occurrence is extremely rare.2,4) The clinical picture of ISH often comprises a falx syndrome characterized by monoparesis of the lower extremity, or hemiparesis in which the leg is weaker than the arm.9,10) Other symptoms may include headache, vomiting, seizures, dementia, language disturbance, oculomotor nerve palsy, or loss of consciousness.5,13) One case of ISH and tentorial hemorrhage presenting with visual disturbances has been reported.19) Although most patients with ISH exhibit neurological deficits at the initial presentation, the appearance of such deficits may be delayed by a few hours to 10 days.6,13,17) And there is no consensus on the ideal treatment of ISH.
We recently treated a patient with ISH who presented with delayed falx syndrome and sensory and visual disturbances. We present this case of ISH and review the clinical presentation and management.
A 66-year-old woman reported a minor head trauma as a result of a fall and was admitted first to another hospital. On admission, her neurological examination showed no abnormality except headache. Initial nonenhanced computed tomography (CT) showed a left frontal ISH (Figure 1). Two days after she was noted to have marked right hemiparesis, especially in the right lower extremity. Nonenhanced CT obtained after development of the right hemiparesis demonstrated an increase in the size of the frontal ISH and a newly developed occipital ISH (Figure 2). She underwent conservative treatment, which did not improve the right hemiparesis. On 13 day after the trauma, she transferred to our hospital. Diffusion magnetic resonance images performed on arrival at our hospital showed no changes in the ISH and no infarction (Figure 3). Upon admission to our hospital, she was alert and oriented, but she reported headache and dizziness. Neurological examination showed mild motor type dysphasia, right homonymous hemianopsia, right hemiparesis, and right hypesthesia. The right lower extremity demonstrated more severe deficits than the right upper extremity in all sensory perceptions (pain, temperature, position, and vibration) and motor power. Deep tendon reflexes of the right extremity were increased, especially in the lower extremity. She was treated with dexamethasone 4 mg intravenously four times daily and 15% mannitol 100 cc intravenously two times daily for three days. Twelve hours after the start of the mannitol and dexamethasone therapy, the patient showed marked improvement in the motor weakness and sensory, visual, and language disturbances. CT obtained 20 days after the trauma showed partial resolution of the ISH (Figure 4). Five months after the trauma, she showed no neurological deficits.
The most likely pathogenic mechanism of posttraumatic ISH is linked to traumatic venous tearing frequently involving the parasagittal bridging veins, which are stretched by the tangential forces that develop after frontal or occipital impact.2,11,14,15) The mechanism principally responsible for laceration of the bridging veins is the linear acceleration provoked, in most cases, by a frontal or occipital impact. Posttraumatic ISH is commonly caused by a mechanism of 'inertia' rather than fracture or cerebral contusion by direct impact3); this theory is consistent with the lack of fracture in most published cases.3) Our patient had neither contusion nor fracture.
ISH commonly develops in front of the posterior half of the falx cerebri but may extend anteriorly and/or reach the subtemporo-occipital area above the tentorium cerebelli.7) Our patient presented with visual disturbances and contralateral hemiparesis. In our patient, the mass effect of the ISH located in the interhemispheric space adjacent to the occipital lobe produced homonymous hemianopsia. The ISH located in the interhemispheric space adjacent to the central sulcus produced contralateral hemiparesis, which was more severe in the lower than in the upper limb, and sensory disturbances.
One patient with ISH and tentorial hemorrhage presenting with visual disturbances has been reported.19) In most cases of ISH presenting with falx syndrome, the ISH is large.18) Our patient did not show a subarachnoid hemorrhage or cerebral contusion, and the size of the ISH was moderate. This suggests that even a moderate-size hematoma can produce the syndrome if the hematoma is located adjacent to the central sulcus or occipital lobe. We think that the incidence of visual disturbance associated with ISH may be higher than that reported previously. Many physicians and patients may neglect these visual symptoms associated with ISH and focus on the motor weakness in the lower limb.
The overall mortality rate of ISH is 24-27%2,14) and is independent of the choice of treatment, indicating that one treatment type (surgical or conservative treatment) is not inherently better9,14) Those supporting surgical intervention insist that an ISH can have a grave prognosis because of the lack of specific signs and the potential for rapid deterioration of consciousness. These clinicians suggest that prompt surgery via a craniotomy is the only way to deal with this condition safely.8,12,13,16) However, some patients with an ISH have been managed successfully with conservative measures in the acute phase.2,5,10,14)
Although there is no consensus on the ideal management of these hematomas, a flexible treatment plan should be developed. The plan should reserve surgical treatment for patients with pronounced symptoms or neurological deficits. The present state of knowledge gives no definitive indication for surgery in the management of an ISH, with the exception of patients with a large hematoma or rapidly deteriorating level of consciousness despite medical treatment.9) An ISH is frequently small and often does not require surgical evacuation. In such cases, the natural history of the ISH tends toward spontaneous reabsorption and recovery of the neurological deficits, which can be followed by serial CT scans. Conservative treatment may be best for patients who are neurologically stable or have concurrent risk factors.2)
Figures and Tables
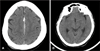 | FIGURE 1Initial computed tomography scans demonstrated a small hematoma in the frontal interhemispheric space (A) and no hemorrhage in the occipital interhemisp-heric space (B). |
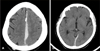 | FIGURE 2Computed tomography scans obtained two days later. A: Frontal image showed enlargement of the frontal interhemispheric subdural hematoma (IHS). B: Occipital image showed extension of the hematoma to the left occipital interhemispheric space. |
References
1. Aring CD, Evans JP. Aberrant location of subdural haematoma. Arch Neurol Psychiatry. 1940; 44:1296–1306.
2. Bartels RH, Verhagen WI, Prick MJ, Dalman JE. Interhemispheric subdural hematoma in adults: case reports and a review of the literature. Neurosurgery. 1995; 36:1210–1214.
3. Delfini R, Santoro A, Innocenzi G, Ciappetta P, Salvati M, Zamponi C. Interhemispheric subdural hematoma (ISH). Case report. J Neurosurg Sci. 1991; 35:217–220.
4. Friedman MB, Brant-Zawadzki M. Interhemispheric subdural hematoma from ruptured aneurysm. Comput Radiol. 1983; 7:129–134.

5. Fruin AH, Juhl GL, Taylon C. Interhemispheric subdural hematoma. Case report. J Neurosurg. 1984; 60:1300–1302.
6. Glista GG, Reichman OH, Brumlik J, Fine M. Interhemispheric subdural hematoma. Surg Neurol. 1978; 10:119–122.
7. Houtteville JP, Toumi K, Theron J, Derlon JM, Benazza A, Hubert P. Interhemispheric subdural haematomas: seven cases and review of the literature. Br J Neurosurg. 1988; 2:357–367.

8. Kasdon DL, Magruder MR, Stevens EA, Paullus WS Jr. Bilateral interhemispheric subdural hematomas. Neurosurgery. 1979; 5:57–59.

9. Llamas L, Ramos-Zúñiga R, Sandoval L. Acute interhemispheric subdural hematoma: two case reports and analysis of the literature. Minim Invasive Neurosurg. 2002; 45:55–58.

10. Ogsbury JS, Schneck SA, Lehman RA. Aspects of interhemispheric subdural haematoma, including the falx syndrome. J Neurol Neurosurg Psychiatry. 1978; 41:72–75.

11. Okamoto J, Ban M, Sakamoto M, Takasugi S, Matumoto K. [Acute interhemispheric subdural hematoma--report of a case (author's transl)]. No Shinkei Geka. 1982; 10:209–213.
12. Pozzati E, Gaist G, Vinci A, Poppi M. Traumatic interhemispheric subdural hematomas. J Trauma. 1982; 22:241–243.

13. Psaltis A, Lath R, McDonald M. Acute interhemispheric subdural haematoma. J Clin Neurosci. 2004; 11:546–548.

14. Rapanà A, Lamaida E, Pizza V, Lepore P, Caputi F, Graziussi G. Inter-hemispheric scissure, a rare location for a traumatic subdural hematoma, case report and review of the literature. Clin Neurol Neurosurg. 1997; 99:124–129.

15. Romano VA, Toffol GJ. Confirmation of traumatic interhemispheric subdural hematoma by magnetic resonance imaging. J Emerg Med. 1994; 12:369–373.

16. Russell NA, del Carpio-O'Donovan R, Mallya KB, Benoit BG, Belanger G. Interhemispheric subdural hematoma. Can J Neurol Sci. 1987; 14:172–174.

17. Seelig JM, Greenberg RP, Becker DP, Miller JD, Choi SC. Reversible brain-stem dysfunction following acute traumatic subdural hematoma: a clinical and electrophysiological study. J Neurosurg. 1981; 55:516–523.
18. Urculo E, Martinez L, Gereka L, Olasagasti V, Olascoaga J, Urcola J. The spontaneous reabsorbtion of posttraumatic interhemispheric subdural haematoma. Acta Neurochir (Wien). 1996; 138:776–777.

19. Yoo JS, Hu C, Hong SK, Kim HJ, Han YP. Clinical analysis of interhemispheric subdural hemorrhage and tentorial hemorrhage. J Korean Neurosurg Soc. 1991; 20:13–19.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download