Abstract
Objective
Our point of interest was focused on the relationship between prognosis and radiographic findings in brain computed tomography (CT) and/or magnetic resonance imaging (MRI) from long-term bed-ridden patients due to polytrauma with head injury who had been followed-up for more than 6 months.
Methods
We had reviewed 47 long-term (>6 months) admitted patients due to polytrauma for 8 years. We retrospectively studied patients' prognosis using clinical parameters such as age, gender, duration of admission, mode of injury, previous medical disease, associated injuries, diffuse axonal injury, space occupying lesion, Glasgow Coma Scale (GCS) score, Glasgow Outcome Scale (GOS) and operative procedures done.
Results
GOS was correlated well with GCS score in polytrauma patients (p<0.001). The operative procedure (decompressive craniectomy) had strong impact on the formation of ventriculomegaly (Evans index≥0.3; p<0.001). The mode of injury also had statistical significance on post-traumatic cerebral infarction (p=0.022).
Though there is lack of a validated consensus in definition, polytrauma is defined as two or more injuries to physical regions or organ systems, one of which may be life-threatening, resulting in physical, cognitive, psychological, psychosocial impairments and functional disability. In another study, polytrauma is defined as a syndrome of multiple injuries of defined severity [injury severity score (ISS) ≥16] with consecutive systemic reactions, which may lead to dysfunction of remote organ, also comprises the complex host response to the injury.9)
Among various types of polytrauma, severe traumatic brain injury (TBI) is classified as Glasgow Coma Scale (GCS) score<9, carries a poor prognosis and the mortality in severe brain injury is approximately 25% with only 20% recovering with a good outcome.11)
The successful management of polytraumatised patients remains challenging despite of expanding scientific knowledge and modern diagnostic and therapeutic approaches. Initial management of the polytrauma patient is of vital importance to minimizing both patient morbidity and mortality. Specific attention is paid to innovations in care and specific controversies in early management as well as local solutions to challenging problems.7)
Our points in this study are focused on the relationship between prognosis and radiographic findings of brain CT and/or MRI from long-term bed-ridden patients due to polytrauma with head injury who had been followed-up for more than 6 months.
Between January 2000 and December 2007, a retrospective study was carried out in 47 patients with polytrauma including head injury who admitted more than 6 months. We performed neurologic examination and brain CT and/or MRI scan was taken within 6 hours after admission. The patients were classified into two groups by initial GCS score (GCS score≥9 as good and GCS score≤8 as bad) and outcome at 6 months post-injury was assessed by using the Glasgow Outcome Scale (GOS)(patient with good recovery and moderate disability as good outcome and patient with severe disability, persistent vegetative state and death as bad outcome). We retrospectively studied using various parameters such as age, gender, duration of admission, mode of injury, previous medical disease, associated injuries, diffuse axonal injury, space occupying lesion, GCS score, GOS and operative procedures done. Statistical analysis was performed using SPSS for windows version 12 (SPSS Inc, Chicago, IL, USA). A p value <0.05 was considered statistically significant.
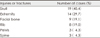
Among the 47 patients, 25 patients (53.2%) were less than 60 years old and 22 patients (46.8%) were exceed 60 years old. There were 36 males and 11 females. Of these, 30 patients (63.8%) were injured by high speed injury such as traffic accident, etc. and 17 patients (36.2%) by low speed injury like fall accident, bicycle injury, etc (Table 1).
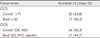
Of 47 patients, various types of intracranial hemorrhage and associated injuries were demonstrated on brain CT and other examinations. traumatic intracerebral hemorrhage 22 cases (21.4%), traumatic subarachnoid hemorrhage 26 cases (25.2%), acute subdural hemorrhage 19 cases (18.4%), acute epidural hemorrhage 9 cases (8.7%), diffuse axonal injury with minimal hemorrhagic contusion 27 cases (26.2%)(Table 2). And associated skull fracture was seen in 19 cases (40.4%), extremities injury 14 cases (29.7%), facial bone fracture 9 cases (19.1%), rib fracture 8 cases (19.0%), pelvic bone fracture 3 cases (4.3%), spine injury 3 cases (4.3%) (Table 3). 37 cases (40.6%) demonstrated ventriculomegaly on follow-up radiographic study. 20 cases (22.0%) demonstrated cerebromalacia. Post-traumatic cerebral infarctions were seen along the vascular territories of the posterior cerebral artery in 5 cases, where anterior cerebral artery in 1 case, basal ganglia in 1 case and multiple cerebral infarctions in 2 cases (Each of the cases was accumulated and included in Table 2, 3, 5).
We classified GCS score and GOS into 2 groups (GCS score≥9 as good and GCS score≤8 as bad; GOS in good recovery and moderate disability were as good and severe disability, persistent vegetative state and death were as bad). Among 47 patients, GCS score of 30 patients were good and that of 17 patients were bad, whereas GOS in 26 patients were good and that of 21 patients were bad. Statistical analysis showed significant correlation between GCS score and GOS (p<0.001)(Table 4).
Among 47 patients, 40 patients underwent operation (decompressive craniectomy 3 cases; shunt procedures 19 cases; both procedures 18 cases). Among the patients with operative procedure, 37 patients (92.5%) showed ventriculomegaly on follow-up imaging, but no patient showed ventriculomegaly who had not undergone any procedures. We found operative procedure (decompressive craniectomy) has strong impact on the formation of ventriculomegaly (p<0.001)(Table 6).
9 head-injured patients (29.0%) by high-speed injury and 11 patients (68.8%) by low-speed injury had post-traumatic cerebral infarction on follow-up neuroimaging. 22 patients (71.0%) by high-speed injury and 5 patients (31.3%) by low-speed injury had post-traumatic infarction. The mode of injury also had statistical significance on post-traumatic cerebral infarction (p=0.022)(Table 7).
In our study, 4 patients with bad GCS score and 16 patients with good GCS score demonstrated post-traumatic cerebral infarction on follow-up neuroimaging. 13 patients with bad GCS score and 14 patients with good GCS score demonstrated no definite post-traumatic cerebral infarction on follow-up neuroimaging. The effect of GCS score had no definite relationship on the post-traumatic cerebral infarction (p=0.093)(Table 7).
For the classification of polytrauma patients, more than 50 scoring systems have been published. Among these systems, the Revised Trauma Score is a physiological scoring system, with high inter-rate reliability, demonstrated accuracy in predicting death and consists of GCS score, systolic blood pressure and respiratory rate to provide a general assessment of physiologic derangement.2) The other definition of polytrauma implies the use of ISS. The ISS is established medical score to assess trauma severity,1,5) by which Keel and Trentz14) defines polytrauma as ISS≥16.
Metabolic changes after trauma were described more than seven decades ago by Cuthbertson et al.6) and characterize as occurring in two different phases, termed the "ebb" phase and the "flow" phase (Table 8). The "ebb" phase is initiated within minutes after trauma and persists for several hours after the initial insult. It is characterized by a decline in body temperature and oxygen consumption, aimed at reducing post-traumatic energy depletion. However, the brief duration of this phase limits its clinical relevance. The "flow" phase, which occurs after compensation of the state of traumatic-hemorrhagic shock, is associated with an increased metabolic turnover, activation of the innate immune system and induction of the hepatic acute-phase response. This results in an increase of the catabolic state with a significantly increased consumption of energy and oxygen. The authors in this study said appropriate immunonutrition should be started in the intensive care unit, preferably by enteral route, in order to counteract the potentially devastating effects of the massive hypermetabolic state after major trauma.12)
The prognosis of patients with TBI is well documented and is highly correlated to the neurobehavioral sequelae after central nervous system (CNS) damage. Estimates of TBI incidence, severity and cost reflect the enormous losses to individuals, their families and society from these injuries. In one study, cerebral damage was a more common cause of death than multiple organ failure following multiple non-penetrating trauma.8) And in other study, older age, low GCS, absent pupil reactivity and the presence of major extracranial injury predicted poor prognosis.16) However, studies about polytrauma patients' prognosis are indecisive. Our study found no definite prognostic factor, either (Table 6, 7). But, severe trauma is still the most frequent cause of death in people below the age of 40 and in one study, among various types of polytrauma, 69 percent of patients with polytrauma had cerebral injuries, 62% thoracic trauma and 86% fractures (40% open fractures).19)
A study of the injury fatality rate in an adult population was done in the Moscow region.20) Investigators determined that side by side with the severity of injury, the direct causes of death were erroneous diagnosis, ungrounded delay of surgical interventions and insufficient use of necessary medical care. They also showed that the attention of administrative and public health agencies to the material/technical equipment of prehospital and hospital medical service and to the training of medical personnel, was insufficient and that activity on accident prevention was inadequate. Determination of causes will help to create concrete measures to decrease injury fatality.
According to a study on the periodic classification of polytrauma, immediate and early trauma deaths are determined by primary brain injuries or significant blood loss (hemorrhagic shock), while late mortality is caused by secondary brain injuries and failure of host defence.14) Individually adjusted surgical "damage control" and "immune control" are important interactive concepts in polytrauma management and trauma-adjusted surgical techniques are crucial to limit the systemic response known to put remote organs at risk. In the "vulnerable phase" when the patient's defense is rather uncontrolled, only "second look" debridement to minimize a "second hit" is recommended. After stabilization of the patient as indicated by improvement of tissue oxygenation, coagulation and decreased inflammatory mediators, "reconstructive surgery" can be applied.9) In a study about late death after severe polytrauma, causes of in-hospital deaths are head injury (37%), adult respiratory distress syndrome (14%), sepsis (11%), hemorrhagic shock (10%), pneumonia (9%), multiple organ failure (9%) and others (10%). Causes of death after discharge include cardiovascular diseases (23%), second major trauma (19%), neurologic diseases (16%), suicide (10%), malignancies (6%) and others (26%). The analysis of survival shows a higher mortality for polytrauma compared with the general population group during the first year after the event (p<0.05). Between 2 years and 10 years after the event, the annual mortality of the polytrauma group approximates the general population group.18) But, elderly patients with minor injuries and pre-existing medical conditions have an increased risk of death relative to their younger counterparts and are more likely to die of medical complications late in their hospital admission.4)
About outcome after polytrauma, despite differences in injury pattern and severity of injury, there is strong evidence from the literature that the quality of life is significantly impaired after major trauma. This is true for functional outcome as well as for psycho-social outcome in up to 70% of patients.3)
In a recent retrospective study by Jiang et al.13) eight hundred forty-six cases of severe TBI (GCS≤8) were analyzed to clarify the effects of multiple factors on the prognosis of patients. At 1 year after injury, the outcomes in these cases were as follows: good recovery (31.56%), moderate disability (14.07%), severe disability (24.35%), vegetative status (0.59%) and death (29.43%). The outcomes were strongly correlated (p<0.05) with GCS score, age, pupillary response and size, hypoxia, hyperthermia and high intracranial pressure (ICP). The authors in this study reported these findings indicated that prevention of hypoxia, control of high ICP and prevention of hyperthermia might be useful means for improving the outcome of patients with severe head trauma (SHT). In our study, GCS score and GOS were divided into two groups and statistical analysis showed significant correlation with each other (p<0.001)(Table 4).
According Neugebauer et al.17) isolated SHT or SHT in combination with polytrauma are important factors for the prognosis of morbidity and mortality in patients suffering from the consequences of accidents. The prognosis mainly depends on the presence of primary mechanical brain injury and the development of secondary brain damage. Causes for the development of secondary brain damage are the intracranial space demand after traumatic injury and edema formation which may result in ischemia, as well as inflammatory processes. Both isolated SHT and polytrauma with or without brain damage may result in a systemic inflammatory response syndrome (SIRS) due to the synthesis of cytokines and other inflammatory mediators which may cause a single or multiple organ failure. Often the organism is able to survive isolated traumatic injuries and functional disturbances, but in combination or accumulation they may be lethal. The hypermetabolism after SHT is often regarded as an interaction between the CNS and the whole organism by the activation of the neuroendocrine axis. In contrast to the consequences of SHT for the whole organism, multiple injuries after polytrauma may affect brain functions, such as the shock dependent disturbance of the brain perfusion accompanied by brain hypoxia which may lead to an aggravated prognosis. Moreover, coagulation, metabolism and fracture healing are influenced by the onset of SIRS as well. Their knowledge about the bidirectional inflammatory interaction between brain and whole organism is still limited. In their study, the effects of secondary surgical interventions which may additionally, stress a traumatized body have to be considered and are the subject for actual clinical discussions and experimental studies.
SHT in combination with polytrauma patients show a different functional, neuropsychological and social outcome depending on several clinical parameters. In a stepwise regression analysis age, injury severity, GCS score, length of coma and weaning time are proved to be suitable predictors. During the intensive care stay an early prognosis on SHT patients is possible.15)
The rehabilitation outcome of patients with severe TBI is well documented and is highly correlated to the neurobehavioral sequelae of CNS damage. However, many of these patients suffer from polytrauma involving systems other than the CNS and to systems involved in acquisition of external information. In the present series of 328 patients with severe TBI, 58% had associated trauma, mostly in the skeletal system. The presence of one single associated trauma had no additional effect on rehabilitation as evaluated by actual work placement. In contrast, multiple lesions were liked with a less favorable outcome, probably due to a greater severity of the initial CNS damage. Disturbances in the various information-acquiring systems (e.g., disturbances in eye movements, visual field defects and severe bilateral auditory deficits) were associated with poor outcome. Presence of peri-articular new bone formation and communicating hydrocephalus, usually associated with prolonged periods of unconsciousness, indicated a poor rehabilitation outcome as well.10)
Though there is obvious lack of information about prognosis of patients with polytrauma including brain injury, we found some statistical significance in our study. Firstly, GOS correlates well with GCS score in these polytrauma patients. Secondly, the operative procedures (decompressive craniectomy and/or shunt) have strong impact on the formation of ventriculomegaly. Lastly, the mode of injury also has statistical significance on post-traumatic infarction. However, there is still lack of randomized trials showing the prognosis of polytrauma patients and therefore more randomized future studies seems justified.
Figures and Tables
References
1. Baker SP, O'Neill B, Haddon W Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974; 14:187–196.
2. Bilgin NG, Mert E, Camdeviren H. The usefulness of trauma scores in determining the life threatening condition of trauma victims for writing medical-legal reports. Emerg Med J. 2005; 22:783–787.

4. Clement ND, Tennant C, Muwanga C. Polytrauma in the elderly: predictors of the cause and time of death. Scand J Trauma Resusc Emerg Med. 2010; 18:26.

5. Copes WS, Champion HR, Sacco WJ, Lawnick MM, Keast SL, Bain LW. The injury severity score revisited. J Trauma. 1988; 28:69–77.

6. Cuthbertson D, Angeles Valero Zanuy MA, León Sanz ML. Post-shock metabolic response. Lancet. 1942; 239:433–437.
7. D'Amours SK, Sugrue M, Deane SA. Initial management of the poly-trauma patient: a practical approach in an Australian major trauma service. Scand J Surg. 2002; 91:23–33.
8. Dereeper E, Ciardelli R, Vincent JL. Fatal outcome after polytrauma: multiple organ failure or cerebral damage? Resuscitation. 1998; 36:15–18.

9. Gebhard F, Huber-Lang M. Polytrauma--pathophysiology and management principles. Langenbecks Arch Surg. 2008; 393:825–831.

10. Groswasser Z, Cohen M, Blankstein E. Polytrauma associated with traumatic brain injury: incidence, nature and impact on rehabilitation outcome. Brain Inj. 1990; 4:161–166.

11. Gunetilleke B. A patient with polytrauma including a severe head injury, haemothorax and cardiac tamponade. Sri Lankan J Anaesthesiol. 2009; 17:80–86.

12. Hasenboehler E, Williams A, Leinhase I, Morgan SJ, Smith WR, Moore EE, et al. Metabolic changes after polytrauma: An imperative for early nutritional support. World J Emerg Surg. 2006; 1:29.

13. Jiang JY, Gao GY, Li WP, Yu MK, Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J Neurotrauma. 2002; 19:869–874.

15. Lehmann U, Steinbeck K, Gobiet W, Regel G. [Prognosis of polytrauma patients with severe craniocerebral trauma during the critical care phase]. Langenbecks Arch Chir Suppl Kongressbd. 1996; 113:340–341.
16. MRC CRASH Trial Collaborators. Perel P, Arango M, Clayton T, Edwards P, Komolafe E, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008; 336:425–429.

17. Neugebauer E, Hensler T, Rose S, Maier B, Holanda M, Raum M, et al. [Severe craniocerebral trauma in multiple trauma. An assessment of the interaction of local and systemic mediator responses]. Unfallchirurg. 2000; 103:122–131.
18. Probst C, Zelle BA, Sittaro NA, Lohse R, Krettek C, Pape HC. Late death after multiple severe trauma: when does it occur and what are the causes? J Trauma. 2009; 66:1212–1217.

19. Regel G, Lobenhoffer P, Grotz M, Pape HC, Lehmann U, Tscherne H. Treatment results of patients with multiple trauma: an analysis of 3406 cases treated between 1972 and 1991 at a German Level I Trauma Center. J Trauma. 1995; 38:70–78.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download