Abstract
Objective
Barbiturates have been demonstrated to reduce intracranial pressure (ICP), but adverse effects, which include hypotension and a long recovery time, make clinical applications difficult. Propofol is also known to have same effect. In the present study, we undertook coma therapy using propofol or barbiturate and compared clinical value in the practical point.
Methods
From June 2004 to April 2007, 38 patients with severe traumatic brain injury underwent thiopental or propofol coma therapy for the 3 days following neurosurgery. Seventeen patients were treated with thiopental (group A) and 21 patients with propofol (group B).
Results
Mean doses were 6.1 mg/kg/hr of thiopental and 4.4 mg/kg/hr of propofol. In group A, mean bispectral indexes were 29.4 on day 1, 27.4 on day 2, and 26.0 on day 3, and in group B, 31.4 on day 1, 29.9 on day 2, and 27.8 on day 3. Mean ICP was 16.8 cm on day 1, 24.4 cm on day 2 and 15.1 cm on day 3 in group A, and 18.3 cm on day 1, 25.4 cm on day 2 and 18.0 cm on day 3 in group B. To maintain systemic normotentsion, dopamine was infused continuously at mean doses of 10.2 µg/kg/min in group A and 4.4 µg/kg/min in group B. Mean times to stationary state were 32.9 hours in group A and 6.6 hours in group B.
The potential neuroprotective effects of barbiturates and propofol have been long recognized. These drugs induce a dose-dependent reduction in the cerebral metabolic rate of oxygen (CMRO2) until silence on electroencephalography (EEG), after which additional doses have no beneficial effects.10,17) Furthermore, they can reduce cerebral blood flow (CBF) in the non-ischemic brain by increasing vascular resistance and reduce intracranial pressure (ICP).8,9)
Numerous theories have been proposed to explain the ability of barbiturates and propofol to prevent or treat cerebral ischemia. The most quoted major neuroprotective mechanism involves the dose-dependent diminution of CMRO2 (up to 50 percent) in human and animals until EEG silence.10,17) Other potential mechanisms of neuroprotection include: stabilization of lysosomal membranes, the attenuation of intracellular calcium concentrations, antioxidative and anti-excitotoxic processes.15,18,19)
The general brief is that maximal neuroprotection is achieved at doses producing silent EEG.16,21) However, higher doses can cause many complications in clinical practice. Moreover, smaller doses may be as efficacious as those required to obtain burst suppression. This is important when considering the deleterious side effects of barbiturates, because of: 1) cardiopulmonary dose-dependent depressant effects and the prolonged ventilation required in patients receiving burst-suppression doses, 2) the increased risk of infection attributable to barbiturates and the need for invasive monitoring, and 3) a depression of mental status that obscures neurological evaluations.9)
However, propofol has a shorter recovery time and induces less systemic hypotension than barbiturate. Therefore, coma therapy using propofol might be more convenient and less complicated than therapies based on barbiturate. In an attempt to compare the clinical application of two drugs, such as depth of anesthesia, changes of ICP, methods to maintain systemic normotension, recovery time from coma therapy and their complications, we performed retrospective study of coma therapy after craniotomy or craniectomy for severe traumatic brain injury (TBI) using propofol or thiopental as general anesthetic agents in two patient groups.
From June 2004 to April 2007, 38 patients underwent coma therapy after brain surgery for the severe TBI at our institute. Of these, 17 patients (group A) underwent coma therapy with thiopental (Pentotal Sodium®) after brain surgery for the first 18 months (from June 2004 to November 2005), whereas, 21 patients (group B) underwent coma therapy with propofol (Fresofol 2%®) during the next 17 months (from December 2005 to April 2007).
Based on preoperative consciousness of patients and intraoperative findings, the following conditions were considered before deciding to perform neuroprotective coma therapy: 1) in severe TBI patients with Glasgow Coma Scale (GCS) score <9; 2) in the case of that severe brain swelling and midline shifting may lead to cerebral herniation; 3) in the case of that discolarization of brain and weak or absent brain pulsation of the operative findings.
After brain surgery, all the patients were transfer to a neurosurgical intensive care unit under a deep hypnotic state. The induction of coma therapy and anesthetic agent titration targeted the appearance of EEG burst-suppression patterns.
EEG signals were obtained using gold cup electrodes applied to the scalp with cream, and were located according to the international 10-20 systems. Skin impedance was maintained at <5 KΩ. The following leads were recorded: left and right frontal-mastoid (FP1-A1, FP2-A2, channels 1 and 2), left and right frontal-Cz (FP1-CZ, FP2-CZ, channels 3 and 4), plus a ground electrode placed at the center of the forehead. EEGs were recorded using a portable Aspect A-1000 EEG monitor (Aspect Medical System, Inc., Natick, MA, USA).
After achieving a burst-suppression pattern on the portable EEG, we replaced electrodes for the Bispectral Index (BIS)™ (BisSensor; Aspect Medical Systems, Inc., Newton, MA, USA) on the forehead, as specified by the manufacturer, and measured signals using an Aspect A-2000 EEG monitor (software version 3.3; Aspect Medical System, Inc., Newton, MA, USA). For polysomnography, we placed a midline frontoparietal electrode for EEG, an electrode at canthus and above unilateral eye for electro-oculography and a submental electrode for electromyography. BIS scores on the monitor were recorded at 2-hours interval using a monitor and data were collected. And then we compared the scores between two groups.
In group A, thiopental was administered in increments of 50-100 mg intravenously at 1- to 5-minutes intervals, and in group B, propofol was administered in increments of 10-20 mg intravenous push at 5- to 10-minutes intervals in an attempt to achieve an EEG burst-suppression pattern. BIS scores were then recorded. Anesthetic doses were incrementally and slowly adjusted upward to achieve the desired endpoint, i.e., an EEG burst-suppression pattern, and BIS scores were then recorded.
After the induction of general anesthesia and loss of consciousness (no response to verbal commends, loss of eyelid reflex, and loss of self respiration), respiration was conducted using a mechanical ventilator with 30-40% oxygen via an endotracheal tube. No other neuromuscular blockers were used. After determining target BIS scores below 40 with silence on EEG, to maintain general anesthesia, patients in group A received thiopental and those in group B propofol at doses of between the previously determined target BIS score +5 and -5.
Patient's anesthetic states were clinically monitored using the modified Observer Assessment of Alertness and Sedation (MOAAS) scale, i.e., when a patient responded readily to his/her name spoken in normal tone a score of 5 was allocated; when a patient responded lethargically a score of 4 was allocated; when a patient responded only after calling in a loud voice or repeatedly a score of 3 was allocated; when a patient responded only after mild prodding or shaking a score of 2 was allocated; when a patient did not respond to mild prodding or shaking a score of 1 was allocated; and a score of 0 was allocated when a patient did not respond to a noxious stimulus.
ICP was monitored by use of epidural fiberoptic catheter and sensor that was placed in the space between dura and craniected muscle or scalp. Digitalized numbers on the ICP monitor were recorded every 2 hours.
Vital signs were checked and recorded every hour. When systolic blood pressure fell below 100 mmHg, a catecholamine (e.g., dopamine) was used to raise and maintain systolic blood pressure to at least 100 mmHg; doses administered were recorded.
After the discontinuance of anesthetics, patient consciousness was checked every two hours using the MOAAS and GCS scores. Recovery time was calculated from the time of stopping anesthesia to the point when these scales indicated a neurologically stationary state, which was checked at least 12 times with 2-hours interval. When the patient recovers slowly after a day, the identical results in the 6 times sequential repeated tests was determined as stationary state. When a patient's vital signs, neurological signs, and respiration became stable, mechanical ventilation was stopped. No thiopental or propofol antagonists were administered. And then, the recovery time was compared between two groups.
The T-test, the Chi-square test and Fisher's exact test were used to compare the two groups in terms of baseline characteristics and each parameter for assessment. Results were considered significant when p-values were less than 0.05. Statistical analyses were performed using SPSS version 12.0 (SPSS Institute, Inc, Chicago, IL).
Table 1 summarizes patient characteristics and initial diagnoses. There were 16 male and 22 female patients of mean age of 54.5 years (range: 33-68) when coma therapy was performed. In group A, there were 10 men and 7 women of mean age 55.5 years (range: 37-68), and in group B, there were 9 men and 12 women of mean age 53.7 years (range: 33-68). Initial diagnoses were as follows; 16 acute subdural hemorrhages (7 in group A and 9 in group B), 10 traumatic intracerebral hemorrhage (4 in group A and 6 in group B), 5 diffuse cerebral hemorrhagic contusion (3 in group A and 2 in group B), 3 epidural hemorrhages (2 in group A and 1 in group B), 3 diffuse cerebral edema with diffuse axonal injury (1 in group A and 2 in group B), and 1 other disease.
All the patients underwent craniotomy or craniectomy. The mean overall preoperative GCS score was 6.6 (6.4 in group A and 6.8 in group B) and ranged from 4 to 8. No significant difference was observed between two groups in terms of demographic parameters, diseases, or preoperative GCS scores.
After achieving a burst-suppression pattern on EEG, BIS scores were recorded every 2 hours for 3 days during coma therapy, and average values were calculated (Table 2). For all patients, mean BIS scores were; 29.4 on the first day, 27.4 on the second day, and 26.0 on the last day. In group A, mean BIS scores were 26.9 on the first day, 24.3 on the second day, and 23.7 on the last day. In group B, mean BIS scores were 31.4 on the first day, 29.9 on the second day, and 27.8 on the last day. The two groups had no differences in terms of BIS scores.
Hypnotic depth was checked by physicians, and MOAAS scores were recorded every 2 hours during coma therapy (Table 2). For all patients, mean MOAAS scores were 0.66 on the first day, 0.61 on the second day and 0.39 on the last day. In group A, mean MOAAS scores were 0.24 on the first day, 0.17 on the second day and 0.00 on the last day. In group B, mean MOAAS scores were 1.00 on the first day, 0.95 on the second day, and 0.71 on the last day. In terms of MOAAS scores, no significant differences were found between the two groups and all the patients were well controlled under deep anesthesia.
In the first day of coma therapy, mean ICP for all the patients was 17.5 cmH2O and 16.8 cmH2O in group A and 18.3 cmH2O in group B, respectively. In the second day, mean ICP for all the patients was increased to 24.9 cmH2O and 24.4 cmH2O in group A and 25.4 cmH2O in group B. In the last day, mean ICP for all the patients was decreased again to 16.6 cmH2O and 15.1 cmH2O in group A and 18.0 cmH2O in group B, respectively. No statistical difference was found between two groups in the ICP (Table 2).
In group A, the mean dose of thiopental administered was 6.1 mg/kg/hr (range: 4.5-7.5 mg/kg/hr) and in group B, the mean dose of propofol administered was 4.4 mg/kg/hr (range: 3.5-6.0 mg/kg/hr).
To maintain systemic normotensive state, dopamine was administered to counter cardiac depression due to anesthetics. Except for dopamine, no other agent was administered to increase systemic pressure. The mean overall dose of dopamine administered was 7.0 µg/kg/min (range: 0.0-14.0 µg/kg/min). In group A, the mean dose of dopamine was 10.2 µg/kg/min (range: 8.0-14.0 µg/kg/min) and in group B, 4.4 µg/kg/min (range: 0.0-8.0 µg/kg/min). Significantly less dopamine was administered to maintain systemic pressure in group B than in group A (p=0.000) (Table 2).
The mean overall recovery time was 18.4 hours (range: 4.0-48.0 hours) and summarized on table 2. In group A, it was 32.9 hours (range: 20.0-48.0 hours) and in group B, 6.6 hours (range: 4.0-10.0 hours). It took shorter time to recover from anesthesia for patients who were treated with propofol than those who were treated with barbiturate (p=0.000).
GCS score after stationary condition from coma therapy was not significantly different before coma therapy. The mean overall post-therapeutic GCS score was 6.8 (6.7 in group A and 7.0 in group B) and ranged from 3 to 10. Four patients (23.5%) in group A got worse than before and 4 patients (19.0%) in group B did. Seven patients (41.2%) in group A improved and 11 patients (52.4%) in group B did. However, the difference between two groups did not have statistical significance (p=0.352).
Table 3 presents the complications that occurred during coma therapy. No patient died during therapy. Although all patients experienced side effects to some degree, their complications were minimal and not life-threatening, i.e., systemic hypotension (systolic blood pressure <100 mmHg) occurred in 35 patients (17 in group A and 18 in group B), electrolyte imbalances (hyponatremia, hypokalemia, or hypercalcemia) occurred in 10 patients (4 in group A and 6 in group B), leukocytosis (>10,000/µL) occurred in 7 patients (4 in group A and 3 in group B), appearance of pneumonic consolidation on chest X-ray occurred in 6 patients (3 in group A and 3 in group B), increased erythrocyte sedimentation rate (ESR) (>20 mm/hr) or C-reactive protein (CRP) (>5 mg/L) in 5 patients (3 in group A and 2 in group B), increased serum blood urea nitrogen (BUN) (>25 mg/dL) or creatinine (>1.5 mg/dL) in 4 patients (1 in group A and 3 in group B), acidosis in 4 patients (2 in group A and 2 in group B), arrhythmia by electrocardiography (ECG) in 3 patients (2 in group A and 1 in group B), increased lactate dehydrogenase H (LDH) in 2 patients (0 in group A and 2 in group B) and deep vein thrombosis in 2 patients (1 in group A and 1 in group B). No fever (>38.0℃) was found in any patient. There was no statistical difference in complication between two groups.
A 64-year-old woman visited emergent care unit with acute alteration of mentality after head trauma from fall-down. Her pupils did not response to the light and she had decorticated movement to noxious stimulation. GCS score was 6. On the computed tomographic (CT) scan, crescent-shaped, high-density lesion on the left fronto-temporal area was found. And midline was shifted to the right side and sulci were obliterated. Under impression of acute subdural hemorrhage, she underwent decompressive craniectomy and subdural hemorrhage was removed. In the neurosurgical field, the brain was tense and swelling up severely and decreased brain pulsation and discoloration was found. After brain surgery, we started coma therapy using propofol with a dose of 4 mg/kg/hr to reduce intracranial pressure and lessen the brain damage. Initially, the dose was titrated by use of portable EEG monitoring with confirmation of burst-suppression pattern and then was maintained with a dose of 6 mg/kg/hr. BIS score was ranged from 35 to 50 during coma therapy. Dopamine with a dose of 4 µg/kg/min was administrated to maintain normotensive systemic pressure. During coma therapy, she experienced increase of serum LDH (315 IU/mL), leukocytosis (15,000/µL) and metabolic acidosis (serum pH 7.30). She recovered from coma therapy 6 hours to stationary state after discontinuance of propofol administration. Fortunately, she improved minimally with withdraw movement to pain and pupillary response to the light returned. But she was unable to cooperate with physician and GCS score was 8. Figure 1 shows pre- and immediate postoperative imaging scan, EEG finding and BIS setting.
In this study, we found that propofol coma therapy has a shorter recovery time from general anesthesia, induces less systemic hypotension, and requires less dopamine than thiopental coma therapy. However, no difference was observed between the propofol and thiopental groups in terms of BIS scores, MOAAS scores, changes in ICP, outcome, and side effects.
In general, propofol has similar neuroprotective effects as barbiturate, but also has several advantages.14) First, propofol has a shorter half time and recovery time from anesthesia than barbiturate, which can facilitate early neurological evaluation. Second, the lower systemic hypotension associated with propofol can reduce catecholamine requirements. Third, some authors have suggested that the risk of infection may be lower for propofol than barbiturate. Finally, propofol provides an energy source due to its lipid content. With coincidence, in presenting study, patients who underwent propofol coma therapy had shorter recovery time and lower systemic hypotension and less catecholamine requirement. But, in terms of infection, there was no difference between two groups. Hatch suggested that propofol coma therapy for >2 days has much higher risk of infection.4) In this study of 3 day-schedule coma therapy, as many as six patients gained pneumonic consolidation on chest X-ray without fever.
However, despite the practical advantages of propofol, it is not completely safe, especially when administered at high doses, because at high levels propofol accentuates lactate accumulation and edema formation in cases of cerebral ischemia with hyperglycemia.5) Moreover, propofol infusion syndrome is a rare but often fatal syndrome that was originally described in critically ill children undergoing long-term (>48 hours) propofol infusion at high doses (>4 mg/kg/hr).4) Its features consist of severe metabolic acidosis, rhabdomyolysis, renal failure, and fatal cardiac failure. Moreover, this pathologic condition can be aggravated by the combined use of glucocorticoid and catecholamine. In combination with glucocorticoid, high dose of propofol can exert profound effects on immunity and inflammation.20) In combination with catecholamine, the negative inotropic effects of propofol can increase catecholamine requirement, and thus, create a vicious cycle that results in a progressive myocardial depressive effect.20) For these reasons, high-dose propofol for prolonged period (>48 hours) is not recommended.2,6) Therefore, determination of optimal barbiturate and propofol concentration is of considerable importance.
In this point of view, BIS scores can titrate the optimal dose of barbiturate and propofol until burst-suppression pattern appear on the EEG. In fact, BIS scores were developed to measure the effects of anesthetic agents on the brain.7) Over several years, a large database of high fidelity EEG recordings and clinical recordings from more than 2,000 patients that received a wide variety of anesthetic regimens, were collected by Aspect Medical Systems, Inc. This database contains recorded EEG segments and records of associated clinically derived hypnotic states. According to this setting, BIS score of 65-85 have been recommended for sedation, score of 40-65 have been recommended for general anesthesia, and at the score of lower than 40, cortical suppression becomes discernible in EEG as a burst suppression pattern. Almost patients in this study had the BIS score of lower 40 during coma therapy. Therefore, BIS scores can be used to manage anesthetics effectively and reduce drug usage, reduce recovery time, facilitate higher quality recovery, and reduce the side effects of prolonged anesthesia.1)
In presenting study, we monitored ICP with epidural catheter and sensor and performed coma therapy during continuous 3 days. However, according to guideline by Martin, et al., severe TBI patients were recommended to monitor ICP with ventricular catheter.13) Although true ICP shows a potential to warn against the elevation of ICP, continuous analysis of intracranial pressure adds information to simple recording of mean trend values actually.3) In fact, there are no strict and absolute guideline for coma therapy. In the traditional stair step treatment of intracranial hypertension, barbiturate coma therapy has the most potential capacity to reduce ICP.12) Therefore, many neurosurgeons consider barbiturate coma therapy for the refractory high ICP.11) In the point of limitation of this study, 3-days coma therapies were performed without precise scientific considerations, and further comprehensive studies are needed to confine the duration of therapy and the time of weaning coma therapy.
Barbiturate and propofol coma therapy are known to have similar important neuroprotective roles. However, in spite of the several practical advantages of propofol over barbiturate such as less systemic hypotension during coma therapy and shorter time to stationary state, it is not a completely safe anesthetic agent. The determination of the optimal dose required to maximize its neuroprotective effects and avoid fatal complications is of considerable importance. The findings of the present study suggest that BIS can be used to determine the optimal dose of propofol so as to increase its neuroprotective effects and reduce its side effects during coma therapy.
Figures and Tables
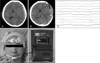 | FIGURE 1A 64-year-old woman visited emergency care unit with acute altered mentality after head trauma. A: At admission, precontrast computed tomographic (CT) image showed crescent-formed, high-density lesion on the left fronto-temporal area and midline shift to right side. B: After craniectomy and removal of hemorrhage, there was aggravated midline shift on the immediate postoperative CT scan. C: In order to titrate propofol dosage, burst-suppression pattern was confirmed by use of the portable EEG monitoring. D: BIS leads were placed on her forehead. E: BIS monitoring was started after titration of propofol dose. EEG: electroencephalography, BIS: bispectral index. |
References
1. Bhargava AK, Setlur R, Streevastava . Correlation of bispectral index and Guedel's stages of ether anesthesia. Anesth Analg. 2004; 98:132–134.

2. Cremer OL, Moons KG, Bouman EA, Kruijiswijk JE, de Smet AM, Kalman CJ. Long-term propofol infusion and cardiac failure in adult head-injured patients. Lancet. 2001; 357:117–118.

3. Czosnyka M, Steiner L, Balestreri M, Schmidt E, Smielewski P, Hutchinson PJ, et al. Concept of "true ICP" in monitoring and prognostication in head trauma. Acta Neurochir Suppl. 2005; 95:341–344.

5. Ishii H, Arai T, Segawa H, Morikawa S, Inubushi T, Kukuda K. Effects of propofol on lactate and edema formation in focal cerebral ischemia in hyperglycemic rats. Br J Anaesth. 2002; 88:412–417.
6. Jacobi J, Fraser GL, Coursion DB, Riker RR, Fontain D, Wittbrodt ET, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002; 30:119–141.

7. Johansen JW, Sebel PS. Development and clinical application of electroencephalographic Bispectrum monitoring. Anesthesiology. 2000; 93:1336–1344.

8. Kochs E, Hoffman WE, Werner C, Thomas C, Albrecht RF, Shulte am Esh J. The effects of propofol on brain electrical activity, neurologic outcome, and neuronal damage following incomplete ischemia in rats. Anesthesiology. 1992; 76:245–252.

9. Levy ML, Rabb C, Couldwell WT, Zelman V, Apuzzo MLJ. Protection of the neural pool. In : Apuzzo MJL, editor. Brain Surgery. Complication avoidance and management. New York: Churchill Livingston;1993. p. 857–889.
10. Michenfelder JD, Theye RA. The effects of anesthesia and hypothermia on canine cerebral ATP and lactate during anoxia produced by decapitation. Anesthesiology. 1970; 33:430–439.

11. Nilsson P, Enbald P, Chamber I, Citerio G, Fiddes H, Howells T, et al. Survey of traumatic brain injury management in European Brain-IT centers year 2001. Acta Neurochir Suppl. 2005; 95:51–53.
12. Robertson C. Critical care management of traumatic brain injury. In : Winn HR, editor. Youmans Neurosurgical Surgery. Vol 5:ed 5. Philadelphia: W.B. Saunders;2004. p. 5103–5144.
13. Rusnak M, Janciak I, Majdan M, Wilbacher I, Mauritz W. Australian Severe TBI Study Investigators. Severe traumatic brain injury in Austria VI: effects of guideline-based management. Wien Klin Wochenschr. 2007; 119:64–71.

14. Skoutelis A, Lianou P, Papageorgious E, Kokkinis K, Alexopolous K, Bassaris H. Effects of propofol and thiopentone on polymorphonuclear leukocyte functions in vitro. Acta Anaesthesiol Scand. 1994; 38:858–862.
15. Smith DS, Rehncrona S, Siesjö BK. Barbiturates as protective agents in brain ischemia and as free radical scavengers in vitro. Acta Physiol Scand Suppl. 1980; 492:129–134.
16. Spetzler RF, Hadley MN. Protection against cerebral ischemia: the role of barbiturates. Cerebrovasc Brain Metab Rev. 1989; 1:212–229.
17. Steen PA, Michenfelder JD. Cerebral protection with barbiturate: relation to anesthetic effect. Stroke. 1978; 9:140–142.
18. Steen PA, Michenfelder JD. Mechanisms of barbiturate protection. Anesthesiology. 1980; 53:183–185.
19. Teichberg VI, Tal N, Goldberg O, Luini A. Barbiturates, alcohols and the CNS excitatory neurotransmission: specific effects on the kainite and quisqualate receptors. Brain Res. 1984; 291:285–292.
20. Vasile B, Rasulo F, Candiani A, Latronico N. The physiology of propofol infusion syndrome; a simple name for a complex syndrome. Intensive Care Med. 2003; 29:1417–1425.
21. Zarchin N, Guggenheimer-Furman E, Meilin S, Ornstein E, Mayevsky A. Thiopental induced cerebral protection during ischemia in gerbilis. Brain Res. 1998; 780:230–236.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download