Abstract
Objective
Chronic subdural hematoma (CSDH) is a relatively common disease with a simple treatment strategy. However, the prognosis for CSDH patient is not always easily determined. The aim of this study was to investigate the prognostic factors for patients with CSDH.
Methods
Forty-six patients who were treated with burrhole trephination surgery for CSDH were retrospectively analyzed. The possible prognostic factors were age, sex, the size of the hematoma, pre-operative midline shifting, density and the location of the hematoma, pre-operative Glasgow Coma Scale (GCS), medical histories, post-operative brain expansion, and post-operative pneumocephalus. Post-operative clinical outcomes were evaluated by modified Rankin Scale (good outcome: mRS 0-1, poor outcome: mRS 2-5).
Results
Among 46 patients, 33 patients (72%) were in the good outcome group while 13 (28%) patients demonstrated a poor outcome. Among variable factors, being older than 65 years, a poor pre-operative GCS (6-12) and a history of diabetes were significantly related to a poor clinical outcome statistically. As a result of logistic regression analysis for these factors, being older than 65 years and a pre-operative GCS under 12 were revealed as independent prognostic factors for CSDH.
Conclusion
Being older than 65 years and a pre-operative GCS under 12 were independent, significant prognostic factors for CSDH. The presence of diabetes was also statistically related with a poor prognosis without a high-risk value. These results could be helpful to predict the prognosis for CSDH after burrhole trephination.
Chronic subdural hematoma (CSDH) is a relatively common disease, which is thought to develop after injury to vessels between the dura and arachnoid after trauma.7,24) Overall mortality with surgical treatment of CSDH is 0-8%.13) Various factors like coagulopathy, pre-operative neurologic condition, patient's age, post-operative pneumocephalus, and brain expansion rates were known as related with prognosis.17) There have been many surgical and non-surgical techniques to approach CSDH treatment. Burrhole trephination and hematoma removal have been widely-used as effective surgical management of CSDH.13,24) However, in some patients, neurosurgeons have encountered neurological deterioration after surgery. We have developed this clinical analysis to assess factors associated with the prognosis for CSDH.
Forty-six patients with CSDH were surgically treated from January 2003 to November 2006 in our institution. All patients underwent simple burrhole trephination and hematoma removal, and a subdural drain catheter was inserted into the hematoma cavity for several days. Careful history taking, neurological examinations, and computed tomography (CT) scans were performed for the initial diagnosis and at post-operative days 1, 7, and 30.
We analyzed not only clinical factors like age, sex, medical histories, coagulopathy, pre-operative Glasgow Coma Scale (GCS), but also radiologic factors like pre-operative amount of hematoma, pre-operative midline shifting, density and location of the hematoma, post-operative brain expansion, and post-operative pneumocephalus. Post-operative clinical outcomes were measured by one of the authors at post-operative day 30 with the modified Rankin Scale (mRS).18)
Coagulopathy was defined as the need for anticoagulant or thrombolytic therapy, a hematological disorder with an increased risk of bleeding, hepatic failure, or hemodialysis.
Patients were divided into the following two groups by age: 1) younger than 65 years and 2) older than 65 years. They were also grouped according to the presence of medical disease(s), such as coagulopathy, diabetes mellitus (DM), or hypertension (HTN), time from trauma to diagnosis (within 30 days, over 30 days ago), air accumulation after the surgery, which was measured by the longest axis of the larger air density on the CT scan at post-operative day 1 (minimal air accumulation below 5 mm, mild accumulation between 5 to 10 mm, moderate accumulation between 11 to 20 mm, and large accumulation over 21 mm), and length of hospitalization (HD) (within a month, over a month). Brain expansion was calculated by comparing the differences between the longest vertical diameter from the inner table of the skull to the brain cortex pre-operatively and at post-operative day 1 (>10 mm, 0-10 mm, <0). Among variable cuts of the CT scan, one of the images was selected that have longest vertical diameter from the inner table of the skull to the brain cortex. We simply comparing the differences of two image cuts.
Pre-operative clinical status was measured by the GCS, and patients were categorized into two groups (GCS under 12, GCS over 13). Patients' outcomes were assessed by mRS at 1 month post-operative day and categorized into two groups (mRS 0-1, mRS 2-5). The amount of hematoma and the air accumulation were measured by one of the authors at post-operative days 1, 7 by CT scan. The longest vertical length from the inner table of the skull to the outer layer of the brain parenchyme on the largest available CT scan image was used as the size of the hematoma, or air accumulation. The clinical and radiological characteristics in all patients are summarized in Table 1.
Categorical variables were compared using the Pearson chi-square test. Factors like age, sex, time interval to diagnosis, pre-operative GCS, hospitalization day, and the presence of coagulopathy, DM, HTN are also analyzed with a Fisher's exact test. Factors related to poor prognosis are analyzed using the logistic regression model. In these models we controlled for and included the following risk factors: patient age (age under 65 compared to age over 65), pre-operative GCS (GCS≧13 compared to GCS≦12) and the presence of DM (no or yes). Consequently, an odd ratios (OR) significantly lower than 1 would indicate that the occurrence would be less likely to occur, whereas an OR significantly greater than 1 would be a valuable prognostic factor for predicting a poor outcome. Ninety-five percent confidence intervals not containing 1 were considered independent prognostic factors. A p-value less than 0.05 was considered statistically significant.
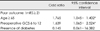
Thirty-two (69.6%) patients were male, and their mean age was 65.2 years (range from 7 to 88 years). A poor post-operative clinical outcome was related to patients older than 65 years (p=0.002).
The mean duration between trauma and the diagnosis was 26.4 days (range from 7 to 90 days), and the mean length of hospitalization was 46.2 days (range from 4 to 243 days). Neither of these factors affected the clinical outcome.
Eight (18%) patients had a history of coagulopathy, and 22 (48%) patients had chronic medical diseases such as DM and HTN. Chi-square analysis revealed that the presence of DM (p=0.025) was related to poor prognosis.
Poor pre-operative clinical status (GCS from 6 to 12) was significantly related to the poor clinical outcome (p=0.002), which had been reported by many authors as an important prognostic factor for CSDH.8,21,25,26) The effects of pre-operative midline shifting, the size of the hematoma, post-operative pneumocephalus, and the degree of brain expansion on the clinical outcome were not statistically significant (Table 2).
Being older than 65, a pre-operative GCS under 12, and the presence of DM were closely related to poor prognosis. However, the odds ratios for DM presence was lower than 1, which could be considered low risk values. The 95% CI for these factors contained 1, and so they were not regarded as independent prognostic factors (Table 3). On the other hand, being older than 65 and pre-operative GCS under 12 carried odd ratios over 1, and the 95% CI indicated that they were independent factors affecting on poor prognosis in patients with CSDH.
CSDH is believed to develop after the tearing of a bridging vein after direct or indirect trauma to the brain.2,4,9) It has many multifactorial prognostic factors such as decreased intracranial pressure, brain atrophy, changes of the skull, and cerebrospinal fluid fistula.13,17) Some non-traumatic factors such as hematological coagulopathy, a clinical history of alcoholism, arteriovenous malformation, anticoagulant therapy, and bleeding tendency can aggravate the clinical outcomes of the disease.1,2)
CSDH is known to be prevalent in two age groups-infants and the elderly, especially those over 75 years old, but can occur in any age group.12) CSDH still occupies a large portion of mortality, despite radiological and surgical improvements. Van Havenbergh et al.25) reported that the morbidity of CSDH with GCS 12 or 13 reached 16.5%, while mortality rates were as high as 6.5%. Ritcher et al.19) and Robinson et al.20) noted that the mortalities of CSDH patients who underwent surgical evacuation were between 1.5% and 8%. These data were very similar to our result of 4%. Old age is known to be an important prognostic factor for CSDH.8) More tensile force on the bridging vein caused by a decrease of total brain parenchymal volume as the patient ages is thought to be the main reason for a high prevalence of CSDH in the elderly. Frequent trauma in the elderly, especially falls, and prevalent anticoagulant therapy can contribute to the high prevalence of CSDH.3,5,6,10) Van Havenbergh et al.25) and Merlicco et al.15) reported that old age and poor pre-operative conditions could affect a patient's outcome. These reports support our data showing that old age (>65 years) independently affects the clinical outcome.
CSDH is known to show a 2-3 times higher incidence in male patients. This is believed to occur not only because of higher head trauma rates in men but also because of the vasoprotective effects of estrogen in women.14,22) In this study, the number of male patients was almost twice that of women, but there was no clinical contribution to the clinical outcome.
Patient's neurological conditions on admission are measured by GCS in this study. Rozzelle et al.21) reported patients with CSDH especially with GCS under 7 had high mortality rates. Weisse et al.26) also recommended early diagnosis and treatment for CSDH because of poor prognosis of patients with GCS under 8. Our study supported this results that poor clinical condition, especially GCS from 6 to 12, not only have had correlation with poor prognosis but also have shown as independent prognostic factor (Table 2, 3).
The pre-operative neurological condition is an index for brain compression by hematoma, and seems to be correlated conversely with the duration to initial diagnosis. Most of the CSDH patients were elderly, and showed mild neurological changes at the early stage of CSDH. This might be the main reason for delayed diagnosis due to mistaking CSDH with dementia, alcoholism, or other diseases.
Poor re-expansion of brain tissue in patients undergoing burrhole trephination and hematoma evacuation with closed drainage is known to be a poor prognostic factor.11) However, in our study, the degree of brain re-expansion was not related to the clinical outcome.
Nagata et al.16) reported that there was no relationship between the amount of air collection in the subdural space after surgery and the prognosis for CSDH. In our study, there was no statistically significant relationship between post-operative pneumocephalus and the clinical outcome.
Patient's old age, pre-operative GCS score, preoperative presence of DM affected the prognosis significantly in this study, and the 95% CI for these factors revealed that age over 65 years and GCS score from 6 to 12 are independent factors affecting poor prognosis in patients with CSDH. This information would be helpful not only to predict the prognosis of the patient, but also to make treatment plans according to these prognostic factors.
Many factors affecting patients' clinical outcomes of CSDH have been analyzed and reported in the literature. However, there have been limited studies about multi-factorial clinical analysis. In this study, the authors recognized important prognostic factors related to the clinical outcome, such as patient age over 65 years, pre-operative neurological condition, and the presence of DM. CSDH is known to be a relatively simple pathological condition with a simple treatment strategy. Nevertheless, its clinical outcome is not so simple, and surgeons may sometimes encounter unpredicted results. In this regard, the prognostic factors would be helpful to estimate the clinical outcomes of CSDH, and to prevent undesirable results.
Figures and Tables
References
1. Drapkin AJ. Chronic subdural hematoma: pathophysiological basis for treatment. Br J Neurosurg. 1991; 5:467–473.

2. Echlin FA, Sordillo SV, Garvey TQ Jr. Acute, subacute, and chronic subdural hematoma. J Am Med Assoc. 1956; 161:1345–1350.

3. Fihn SD, McDonell M, Martin D, Henikoff J, Vermes D, Kent D, et al. Warfarin Optimized Outpatient Follow-up Study Group. Risk factors for complications of chronic anticoagulation. A multicenter study. Ann Intern Med. 1993; 118:511–520.

4. Gardner WJ. Traumatic subdural hematoma with particular reference to the latent interval. Arch Neurol Psychiat. 1932; 27:847–858.

5. Goldberg RJ, Gore JM, Gerwitz JH. The impact of age on the incidence and prognosis of initial acute myoardial infarction: the hertzer heart attack study. Am Heart J. 1989; 117:543–549.
6. Hammermeister KE, Sethi GK, Henderson WG, Oprian C, Kim T, Rahimtoola S. Veterans Affairs Cooperative Study on Valvular Heart Disease. A comparison of outcomes in men 11 years after heart-valve replacement with a mechanical valve or bioprosthesis. N Engl J Med. 1993; 328:1289–1296.

7. Hancock DO. Cerebral collapse associated with chronic subdural hematoma in adults: a comparison of two methods of treatment. Lancet. 1965; 1:633–634.
8. Yamamoto H, Hirashima Y, Hamada H, Hayashi N, Origasa H, Endo S. Independent predictors of recurrence of chronic subdural hematoma: results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003; 98:1217–1221.

9. Jamieson KG, Yelland JD. Surgically treated traumatic subdural hematomas. J Neurosurg. 1972; 37:137–149.

10. Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982; 306:1018–1022.
11. Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo). 2001; 41:371–381.

12. Kotwica Z, Brzeziński J. Chronic subdural haematoma treated by burr holes and closed system drainage: personal experience in 131 patients. Br J Neurosurg. 1991; 5:461–465.

14. Mellergard P, Wiston O. Operations and re-operations for chronic subdural haematomas during a 25-year period in a well defined population. Acta Neurochir (Wien). 1996; 138:708–713.
15. Merlicco G, Pierangeli E, di Padova PL. Chronic subdural hematomas in adults: prognostic factors. Analysis of 70 cases. Neurosurg Rev. 1995; 18:247–251.

16. Nagata K, Asano T, Basugi N, Takara K. Studies on the operative factors affecting the reduction of chronic subdural hematoma with special reference to the residual air in the hematoma cavity. No Shinkei Geka. 1989; 17:15–20.
17. Obana WG, Pitts LH. Management of head injury. Extracerebral lesions. Neurosurg Clin N Am. 1991; 2:351–372.
18. Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J. 1957; 2:200–215.

19. Richter HP, Klein HJ, Schäfer M. Chronic subdural haematomas treated by enlarged burr-hole craniotomy and closed system drainage. Retrospective study of 120 patients. Acta Neurochir (Wien). 1984; 71:179–188.

20. Robinson RG. Chronic subdural haematoma: surgical management in 133 patients. J Neurosurg. 1984; 61:263–268.
21. Rozzelle CJ, Wofford JL, Branch CL. Predictors of hospital mortality in older patients with subdural hematoma. J Am Geriatr Soc. 1995; 43:240–244.

22. Sambasivan M. An overview of chronic subdural haematoma: experience with 2300 cases. Surg Neurol. 1997; 47:418–422.
23. Scotti G, Terbrugge K, Melançon D, Bélanger G. Evaluation of the age of subdural haematomas by computed tomography. J Neurosurg. 1977; 47:311–315.
24. Tabaddor K, Shulman K. Definitive treatment of chronic subdural hematoma by twist-drill craniotomy and closed-system drainage. J Neurosurg. 1977; 46:220–226.
25. van Havenbergh T, van Calenbergh F, Goffin J, Plets C. Outcome of chronic subdural haematoma: analysis of prognostic factors. Br J Neurosurg. 1996; 10:35–39.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download